Golden Root (Rhodiola Rosea and Rhodiola integrifolia)
This is the 25th post in the Stacking Functions in the Garden, Food Forest and Medicine Cabinet : The Regenerative Way From Seed To Apothecary series.
This post serves as the 25th post which is part of the above mentioned (Stacking Functions in the Garden, Food Forest and Medicine Cabinet : The Regenerative Way From Seed To Apothecary series).
These two species are totally underestimated and under-utilized in food forest design and regenerative mountain or ocean garden design and we need to change that!
We are talking about plant so tough they can grow on the side of a cliff in the high alpine in the Rockies, Himalayas and Alps or in the arctic tundra on Baffin Island or in Siberia. These plants grow on the rocky ocean shores and cliffs in Nova Scotia and Newfoundland, Iceland, Norway and Russia.
Huge potential for northern food forest, regenerative ocean gardeners and inland cold climate/high altitude regenerative garden designers here.
I have seen this stuff growing and thriving up at 7500 feet above sea level in the Cascade Mountains in BC and I know people that grow it in the Yukon.
This adaptogen that hails from within the arctic circle, can be grown in standard garden conditions up to zone 7 in the shade/part shade (or in the sun if Zone 4 or less or if at high altitude).
These hardy succulent perennials are extremely long lived and can live up to 75 years! The plants can be harvested and divided throughout that time frame allowing for both access to powerful medicine, and propagating new plants via cloning while allowing the mother plant to remain healthy and produce for decades.
This plant sells currently for $181.50 per lb for organically grown dried plant material here in Canada.
As you will see below, the benefits this potent adaptogen offer in enhancing human health may be worth even more to you than the potential of selling your harvests for big bucks.
Rhodiola is a genus of medicinal plants that originated in Asia and Europe and are used traditionally as adaptogens, antidepressants, and anti-inflammatory remedies. Rhodiola plants are rich in polyphenols, and salidroside and tyrosol are the primary bioactive marker compounds in the extracts of Rhodiola rosea.
This plant originated in Asia (Brown, Gerbarg, and Ramazanov, 2002) and has spread into the North Eastern region of the North American continent (NRSC). Research has revealed that Rhodiola rosea contains powerful adaptogens, which has been proven to protect animals and humans from mental and physical stress, toxins and cold. It has also been found to enhance physical and mental performance (Brown, Gerbarg, and Ramazanov, 2002). Large demand has resulted in over harvesting the plant, endangering it in the Eastern U.S.A., and areas in Asia (NRSC & Platikanov and Estatieva). Regenerative home or farm scale cultivation could help to take stress off of Rhodiola rosea growing in the wild.
Rhodiola rosea L. (golden root) is a herbaceous short-rhizome monopodially growing polycarpic with succulent leaves and an elongated erect shoot, hemicryptophyte. This arctic-alpine species with a wide North American range and disjunctive Eurasian range grows in the mountains of Western Europe, the Balkan Peninsula, Asia Minor, Mongolia, China, East Turkestan, and the Scandinavian countries. A significant part of the range covers the mountains of Southern Siberia: Altai, Western and Eastern Sayan, and the mountains of Tuva and Transbaikal.
Another plant in the Rhodiola family, Rhodiola integrifolia, grows in the Rocky Mountains, and is similar in its medicinal constituents and growing requirements to Rhodiola rosea. They are similar in that they both only grow in northern latitudes, and primarily in alpine ecosystems. They live in areas with similar weather patterns, ground cover content, are both sedums, and have similar flower structures.
Common Names: Rose Root, Rhodiola, Golden root, Arctic root, King's crown, ledge stonecrop
Family: Crassulaceae
Part used for medicine/food: Root and Leaves
Constituents: Phenylpropanoids (rosavin, rosin, rosarin), phenylethanol derivatives (salidroside, rhodioloside), flavonoids, mono & triterpenes, phenolic acid, epicatechin gallate, catechin gallate, and 6′-O-galloylsalidroside
Medicinal actions: Adaptogen, tonic, anti-cancer, stimulant, mental enhancer, anti-stress, antioxidant, anti-proliferative, reproductive health enhancer, immuno-stimulant, osteoprotective and osteoregenerative cardioprotective, radioprotective and neuroregenerative (promotes neurogenesis).

Pharmacology:
Stimulates NE, dopamine, serotonin and nicotinic cholingergic effects in the CNS and enhances effects of those neurotransmitters.
Medicinal Use:
The effects on the nervous system for this herb can be both stimulating and sedating depending on the dose. It can enhance physical endurance, and sexual potency, improves thyroid, thymus and adrenal function, and protects the nervous system, heart and liver through antioxidant effects. It increases the body’s resistance to stress and has a protective effect upon neurotransmitters (especially serotonin and dopamine). It has specific use for headaches in neurasthenia and will enhance cognitive function, learning, memory and concentration.
Also good for Coughs, bronchitis, pneumonia, hemoptysis, hematemesis, shortness of breath, chronic diseases, childhood development, adjunct cancer treatment, leukorrhea, wheezing, hypertension, chest pain, palpitations, longevity, low back pain, arthritis, stamina, stress-related insomnia, general symptoms associated with stress, brain fog, fibromyalgia, mental fatigue, chronic fatigue, muscle recovery, poor appetite, anemia, irritability, headaches, fatigue, used topically to treat blood stagnation from traumatic injury and burns, protects the brain and central nervous system.
Cold Hardiness: 1 to 7
Native Range:
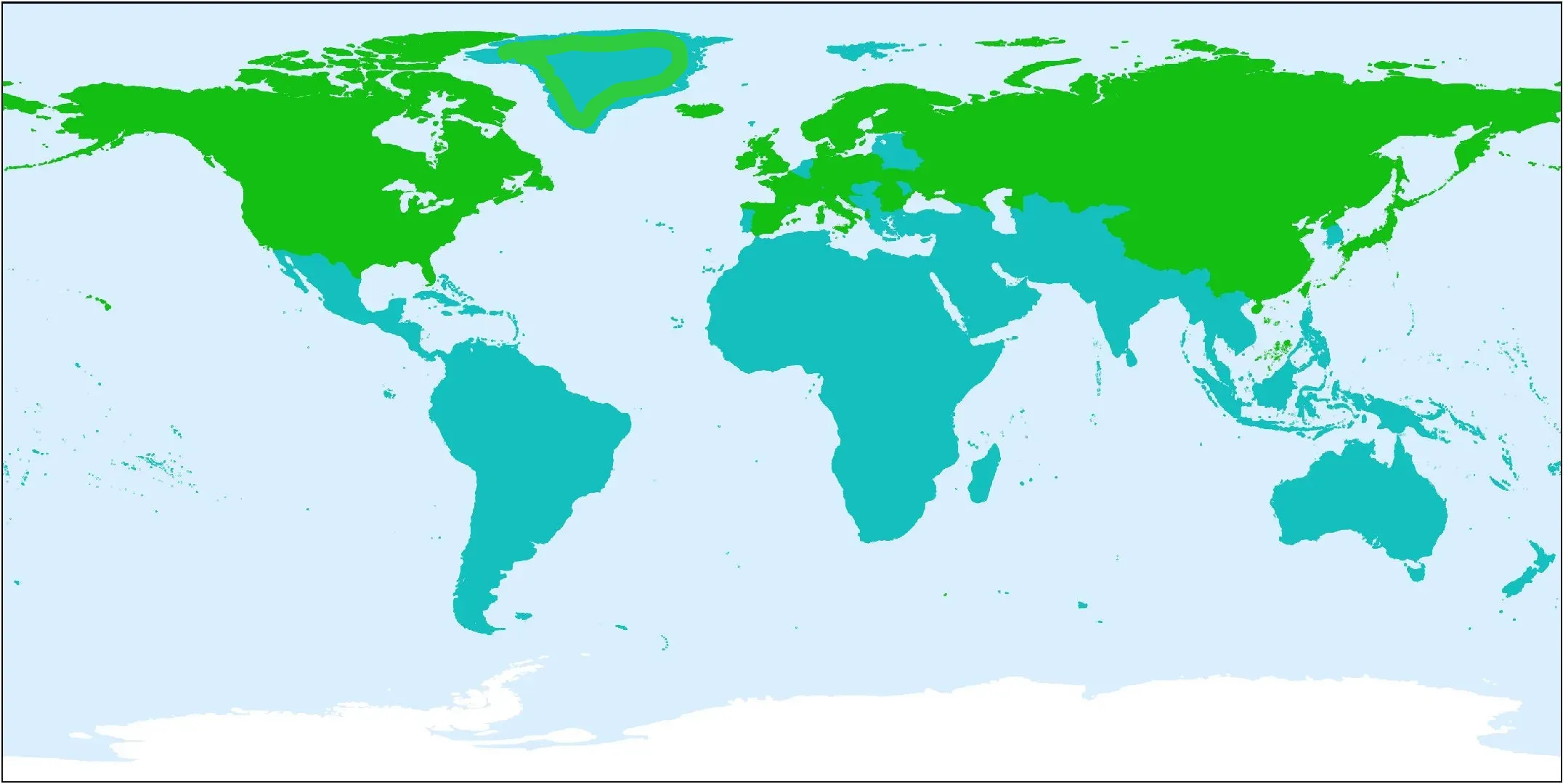
Native to cool temperate and subarctic regions of the Northern Hemisphere, including Europe, Asia, and North America, particularly in high-altitude, mountainous areas.
Europe:
It's found in countries like Albania, Austria, Bulgaria, Finland, France, Germany, Scotland, Ireland, Great Britain, Iceland, Ireland, Italy, Norway, Poland, Romania, Spain, Sweden, Switzerland, and Ukraine.

Rose Root growing on the cliffs on the Isle Of Skye, Scotland (source) Asia:
It occurs in regions of China, Japan, Kazakhstan, Korea, Mongolia and Russia.
North America:
In North America, it is found from Newfoundland and Labrador south to Pennsylvania, and historically in North Carolina.
Specifically, it can be found in Alaska, Maine, New Brunswick, New York, Newfoundland, North Carolina, Nova Scotia, Pennsylvania, Québec, Vermont, and other areas.
Other regions:
It also grows in Greenland, and parts of the Canadian Arctic Archipelago.
Rhodiola integrifolia, known as ledge stonecrop or western roseroot, is native to north-easternmost Russia (including Kamchatka) and western North America, where it thrives in mountainous subalpine and alpine habitats.
Rhodiola integrifolia native range regions:
North America: It's found in mountainous regions from Alaska to California, and east to the Rocky Mountains and Great Lakes region.
Specific US States: Alaska, California, Colorado, Idaho, Montana, Nevada, New Mexico, Oregon, Utah, Washington, and Wyoming.
Canada: Alberta and British Columbia.
Russia: Kamchatka and other areas in north-easternmost Russia.
Growth Form:
Perennial herb that grows from a short, fleshy rootstock, with several stems reaching up to 30-40 cm tall, and has succulent leaves and yellow to greenish-yellow flowers in summer.
Reproduction:
It is a dioecious plant, meaning (as with other species like Cannabis and Ginkgo trees) it has separate male and female plants.


Flowering and Pollination:
Flower reproductive parts: the flower has either only pollen- or only seed-producing parts.
Flowering occurring from May to August.
Light intensity and temperature impact the visitation of the principal pollinators, the Hoverflies and Bumblebees. Population size plays an important role in the mating system and pollination. Populations received more frequent visits by the principal pollinators usually had higher outcrossing rates.
Flower Characteristics: The small, yellow, star-shaped flowers are borne in dense terminal corymb-like heads.
Seed Production: After pollination, the female flowers develop into seed pods, which contain winged seeds.
Habitat and Ecological Niche:
Habitat characteristics
Altitude: Grows at altitudes up to 2,300 meters (7500 feet plus).
Substrate: Grows in moist cliffs, talus ridges, dry tundra, rocky ledges and bald arctic terrain.
Soil: Grows in sand or loam sand, and does best in dry soil
Location: Grows in tundra, slopes, ridges, cliffs, crevices of mountain rocks, on sea cliffs and well drained soil areas with humidity from nearby bodies of water
The species is widespread in the subalpine and alpine mountain belts; along river banks, it descends into the forest belt.
In the forest belt, it grows on avalanche cones and in sparse forests along the banks of streams and rivers. It is most abundant in the subalpine belt on the upper slopes of the valleys. It is encountered in places with prolonged snow cover, along temporary streams, river banks, on stony placers, and rocky outcrops. In the alpine belt, it occurs on moraines, on the slopes.
Health Benefits of Golden Root (Rhodiola Rosea and Rhodiola integrifolia)
Rhodiola improves our resistance to the damaging effects of stress.
From helping the body adapt to stress via adrenal support to supporting healthy immunity, Rhodiola is a very medicinally versatile herb.
While Rhodiola may be one of those herbs that you may not have heard of — yet. But it is a versatile and powerful plant ally and rhodiola benefits include improving hormonal health and reproductive health.
It is an adaptogenic herb with tremendous fat-burning, energy-enhancing and brain-boosting power. Adaptogens including rhodiola are a group of plants that can help your body adapt to physical, chemical and environmental stress. Rhodiola is one of the most effective in this family, due to containing active compounds like rosoavin that have the ability to help balance the stress hormone, cortisol.
This unique herb that is a member of the Rhodiola genera in the Crassulaceae plant family grows at high altitudes in the arctic areas of Asia and Eastern Europe. Rhodiola rosea has been a part of traditional medicine systems practiced across the world, especially in parts of Europe, Asia and Russia, for many centuries.
Historically, rhodiola has a long history of use in Traditional Chinese Medicine, especially for improving stamina and reducing stress. The Vikings also used rhodiola to enhance physical strength, while the Sherpa people used it to climb at high altitudes, even to conquer Mt. Everest.
The Russians have studied rhodiola benefits extensively over the past 70 years, mostly for improving work performance and endurance while fighting insomnia, fatigue, anxiety and depression. It has also been used to cleanse the body, fight cancer and help treat tuberculosis.
What does rhodiola do to the body to help make it more capable of dealing with stress?
As an “ergogenic aid” and an adaptogen— or a “natural herbal product that is non-toxic in normal doses, that produces a non-specific response, and that has a normalizing physiologic influence”— rhodiola is very helpful for improving both physical and mental energy and for fighting the negative effects of stress.
It helps body adapt to stress by decreasing or preventing hormonal changes tied to prolonged stress. Research suggests that some of the ways it does this is by acting on beta-endorphins and opioid neuropeptides to enhance stress tolerance and by positively affecting other stress adaptation factors.
Studies have found that Rhodiola rosea contains more than 40 kinds of chemical compounds. Active constituents found within rhodiola that are responsible for its pharmacological effects include rosavin and salidroside. Rosavin is the only constituent unique to Rhodiola rosea within the Rhodiola plant family, while salidroside is common to most other rhodiola species.
Rosavin is found in higher concentrations than salidrosides, with approximately a 3:1 ratio within Rhodiola rosea. In animal studies, it’s been found that rosavin contributes to rhodiola’s benefits by having antidepressant-like, adaptogenic, anxiolytic-like and stimulating effects.
1. Helps Burn More Belly Fat
One of the many incredible characteristics of rhodiola is that it helps your body burn stored fat more efficiently as fuel. We all know that exercise can increase fat loss, but if you want an extra edge, then consider taking rhodiola along with regularly exercising to accelerate your weight loss efforts.
What allows rhodiola to help lose belly fat? Certain animal studies have found evidence that Rhodiola rosea might reduce visceral white adipose tissue and increase hypothalamic norepinephrine to help prevent diet-induced obesity.
Rhodiola’s most active compound, rosavin, has been shown to trigger a fat-burning response. Because it helps normalize cortisol levels, rhodiola may also reduce cravings for unhealthy “comfort foods” and delay fat-accumulation that is tied to high cortisol levels (especially fat around the abdomen/belly).
Rosavin works by stimulating an enzyme called “hormone-sensitive lipase,” which has the ability to breakdown fat that is stored in adipose tissue (in the belly area). Some sources suggest that if you combine taking rhodiola extract with doing moderate exercise, the breakdown of belly fat increases even more.
2. Increases Energy and Athletic Performance
Research implies that if you’re looking for a natural way to boost energy and increase athletic performance, then rhodiola may be for you. Today, one of the most popular uses of rhodiola is for increasing energy, stamina and strength.
Rhodiola may help increase your stamina and endurance by increasing your red blood cell count and lowering oxidative damage. Red blood cells carry oxygen to muscles, and having a higher count can dramatically improve an athlete’s performance and help to delay fatigue. Rhodiola benefits work by boosting EPO, also known as erythropoietin, which stimulates RBC production.
According to a study published in the International Journal of Sports Nutrition and Exercise Metabolism, rhodiola has anti-inflammatory benefits that aid rapid recovery of muscles and improve endurance. Another study performed on rats found that supplementing with rhodiola could increase endurance by allowing the animals to swim 25 percent longer. The improvements happened because rhodiola was found to increase synthesis of ATP, which is essential for cellular energy.
3. Helps Fight Physical and Mental Fatigue
You don’t have to be an athlete to experience the benefits of rhodiola. Not only can it help reduce physical fatigue, but it may also decrease mental fatigue and symptoms like brain fog or lack of concentration.
Rhodiola is also often used to help people overcome exhaustion from low-intensity, but frequent, exercise or movements. Rhodiola has been shown to increase workplace performance and decrease the effects that sleep deprivation can have on your body.
A 2012 systematic review of 11 randomized, controlled trials that focused on rhodiola’s anti-fatigue effects found that “some evidence suggests that the herb may be helpful for enhancing physical performance and alleviating mental fatigue.” However, further studies are needed.
4. Helps Lower Cortisol
One of the main reasons people turn to adaptogenic herbs like rhodiola is to help balance cortisol levels, which can be beneficial for slowing age-related symptoms and for looking and feeling better. Studies indicate that rhodiola can be helpful for calming your body when your nervous system goes into “fight or flight” mode due to dealing everyday stressors.
When the hormone cortisol stays high for a long period of time, such as from emotional or physical stress, it can cause you to experience stress-related symptoms, such as:
lowered blood glucose response
abdominal weight gain
thyroid issues
hormone imbalance
decreased memory
weakened immunity
By keeping cortisol levels balanced, you can improve your health in multiple ways, especially when it comes to feeling younger and more energized. High cortisol levels over an extended period of time may contribute to accelerated signs of aging, higher levels of psychosocial stress, poorer cognitive performance, atrophy of memory-related structures of the brain, weight gain and exhaustion — exactly the reason why rhodiola may make a helpful anti-aging supplement.
5. Can Help Fight Depression and Improve Brain Function
Another benefit of supplementing with rhodiola is that it’s been shown to help improve cognitive functioning and to help as a depression natural remedy.
Rhodiola may help to increase the sensitivity of your neurons (cells of your brain and nervous system), including the two neurotransmitters serotonin and dopamine. These neurotransmitters are known for increasing focus, memory, pleasure, and overall mood improvement — making them very important for preventing anxiety and depression.
In animal studies, rhodiola has also been shown to help repair damaged neurons in the hippocampus, a region of the brain considered to be the center for emotion, memory and the autonomic nervous system regulation.
Many doctors of functional medicine prescribe rhodiola as an effective natural alternative to anti-depressant medications. This works because rhodiola may increase dopamine sensitivity, which has been shown to improve moods and also to help fight food cravings and addictions.
A small 2015 study that was supported by the National Center for Complementary and Integrative Health (NCCIH) tested rhodiola against the drug sertraline (often prescribed to treat depression) and a placebo in 58 adults with mild-to-moderate major depressive disorder. Results showed that all treatments were similarly effective in reducing depressive symptoms (there were no significant difference found between groups at the end of the study), but the participants who took rhodiola had fewer side effects than those who took sertraline.
Can rhodiola also relieve anxiety? A trial involving 80 “mildly anxious participants” found that compared to controls, the experimental group (taking Rhodiola rosea) demonstrated a “significant reduction in self-reported, anxiety, stress, anger, confusion and depression at 14 days and a significant improvements in total mood.” No relevant differences in cognitive performance between the rhodiola and untreated groups were observed. Rhodiola supplementation was shown to have a “favourable safety tolerability profile.”
Another small pilot study including 10 adults with anxiety found that supplementing with 360 milligrams of rhodiola daily for 10 weeks led to significant improvement in symptoms of generalized anxiety disorder and a reduction in the Hamilton Anxiety Rating Scale scores.
A review of 36 studies concluded that rodiola improves learning and memory function.
One study found that just a single dose of rhodiola increased memory and had an antidepressant effect. It suggested that rhodiola could become a good tool to increase cognition and counteract mood disorders in people.
Another research review concluded that the therapeutic properties of rhodiola benefit many age-related diseases.
6. Anticancer Properties
Salidroside, a potent component of rhodiola, has been investigated for its anticancer properties.
In three separate studies, researchers demonstrated that the compounds in Rhodiola prevented the growth of breast cancer21, colon cancer22, and bladder cancer23 cells. These studies show promise for Rhodiola as a potential cancer treatment and further research points to Rhodiola as a treatment in cancer24.
If you’re currently undergoing cancer treatment, be sure to speak with your doctor before adding Rhodiola or any supplement to your regimen.
7. Rhodiola And Premature Ovarian Failure
Premature Ovarian Failure or Primary Ovarian Insufficiecy (POI) is a condition in which women lose their fertility and period before age 40.
Researchers gave Rhodiola to 40 study participants who had experienced premature ovarian failure. At the end of the study, 25 women had regained their period, and 11 were pregnant.
While this benefit of Rhodiola is not part of the conventional standard of care for fertility treatment, this study helps demonstrate why many traditional herbalists have recommended Rhodiola for fertility.
8. Rhodiola Contains Compounds Which Optimize Mitochondrial Health:
Mitochondria are the powerhouses of your cells, generating energy to fuel your cells’ biochemical reactions. Via optimizing their function and promoting their rates of regeneration within your body you are building a solid foundation for health, vibrancy and longevity from the cellular level up.
Historical records reveal that the Vikings utilized Rhodiola to promote their endurance and physical strength. Modern research suggests that Rhodiola helps healthy energy production and mitochondrial function, benefiting various aspects of health, including athletic performance and healthy stress responses.
Rodent models demonstrate that Rhodiola can activate the synthesis or resynthesis of ATP in the mitochondria and stimulate energy production following intense exercise. This can promote muscle health, cell regeneration, and energy metabolism by increasing the synthesis of ATP, RNA, protein, and amino acids. Moreover, Rhodiola has demonstrated antioxidant properties, helping to attenuate the effects of oxidative stress and promote mitochondrial biogenesis and function.
Here's a more detailed explanation:
Mitochondrial Biogenesis:
Rhodiola, especially Rhodiola rosea, has demonstrated the ability to promote mitochondrial biogenesis, which is the process by which new mitochondria are formed.
Mitochondrial Function:
Rhodiola can also enhance mitochondrial function, which is crucial for energy production within cells.
9. Rhodiola Contains Compounds That Increase The Endogenous Production Of Adult Stem Cells (aka “somatic stem cells”):
Stem cells are the fundamental building blocks of the human body. Cells can differentiate and reproduce any tissue such as heart tissue, muscle, cartilage, bone, or liver. Newborn children have a lot of circulating stem cells that are needed for development and can quickly help them recover from infectious diseases and injuries. As we age, the quantities of circulating stem cells in the body begin to reduce each year, making healing and recovery much more difficult over time. You can increase the production of endogenous stem cells via eating specific superfoods (including ginger) to boost your health and circulating stem cell count.
10. Rhodiola Contains Compounds that offer Osteoprotective and Osteoregenerative benefits:
Rhodiola rosea, particularly its active compound salidroside, has a protective effect on bone health, preventing bone loss and promoting bone formation after injury.
Here's a more detailed look at the evidence:
Salidroside and Bone Metabolism:
Salidroside, a key compound in Rhodiola rosea, has shown promise in modulating bone metabolism.
It can promote osteoblast proliferation and differentiation, which are essential for bone formation.
Salidroside enhances osteogenic activity by increasing alkaline phosphatase activity and mineralization markers.
It also upregulates key regulatory proteins involved in bone formation, such as runt-related transcription factor 2 and osterix.
Studies have shown that salidroside can increase bone mineral density (BMD) in postmenopausal osteoporosis.
It can also restrain osteoclast maturation, which are cells that break down bone tissue, and increase the active osteoblast percentage in bone tissue.
Mechanisms of Action:
Salidroside protects against bone loss by inhibiting oxidative stress and promoting osteogenesis via Nrf2 activation.
It can also facilitate angiogenesis, which is the formation of new blood vessels, through the hypoxia-inducible factor 1-alpha and vascular endothelial growth factor pathway, which is crucial for coupling bone development with vascular support.
Salidroside has antioxidant properties, which can help reduce oxidative stress and promote osteogenic differentiation.
11. Rhodiola Contains Compounds that offer Cardio-Protective and Cardio-Regenerative benefits:
Rhodiola rosea, an adaptogen, exhibits cardioprotective and cardio-regenerative effects, including preventing stress-induced cardiac damage, improving myocardial contractility, and reducing the risk of ischemia-reperfusion injury and arrhythmias.
Here's a more detailed look at the cardioprotective aspects of Rhodiola rosea:
Cardioprotective Effects:
Stress-Induced Cardiac Damage:
Studies have shown that Rhodiola rosea can prevent stress-induced cardiac damage in animals.
Improved Myocardial Contractility:
Rhodiola rosea has been observed to increase contractility in the postischemic period.
Anti-arrhythmic Effects:
Rhodiola rosea exhibits antiarrhythmic activity, which can be attributed to the synthesis of opioid peptides and stimulation of opioid receptors.
Ischemia-Reperfusion Protection:
Rhodiola rosea has demonstrated protective effects in ischemia/reperfusion (IR) and hypoxia-induced cardiomyocyte cell death, potentially preventing IR-induced ventricular tachycardia.
Mitochondrial Protection:
Rhodiola rosea has been shown to be a mitochondrial protectant, enhancing mitochondrial quality and increasing cardiac anti-stress capacity.
Antioxidant Effects:
Rhodiola rosea has antioxidant properties that can help protect cells from damage.
Improved Blood Flow:
Rhodiola formulations can promote blood flow and reduce mean arterial pressure.
Reduced Oxidative Stress:
Rhodiola rosea can enhance antioxidant capacity, thereby reducing oxidative stress.
Underlying Mechanisms:
Adaptogenic Properties:
Rhodiola rosea is considered an adaptogen, meaning it can help the body resist stress and improve overall well-being.
Nrf2 Activation:
Rhodiola rosea can activate Nuclear factor erythroid 2-related factor 2 (Nrf2), which enhances the antioxidant system.
Salidroside:
Salidroside, a key compound in Rhodiola rosea, has been shown to protect human endothelial cells from oxidative damage and increase the expression of HIF-1α, HIF-1β, and VEGF during ischemia and hypoxia.
Limited Adrenergic Effect:
The antistressor and cardioprotective effects of Rhodiola rosea are associated with limited adrenergic effect on the heart.
12. Rhodiola Contains Compounds that offer Radioprotective benefits:
Rhodiola rosea, particularly its constituent rosavin, shows promising radioprotective effects, mitigating radiation-induced injury by modulating inflammatory responses and oxidative stress.
Here's a more detailed explanation:
Radioprotective Effects:
Studies have shown that Rhodiola rosea and its bioactive compounds, including salidroside, herbacetin, rosavin, and arbutin, can improve the cell viability of irradiated IEC-6 cells.
Rosavin's Role:
Rosavin, a key compound in Rhodiola rosea, has been found to significantly improve survival rates and reduce intestinal damage in irradiated rats by modulating inflammatory responses and oxidative stress.
Mechanism of Action:
Rhodiola rosea's radioprotective effects are believed to be linked to its ability to scavenge free radicals and reduce oxidative stress, which are key factors in radiation-induced damage.
Adaptogen:
Rhodiola is considered an adaptogen, a class of natural substances believed to stimulate the body's resistance to physical, environmental, and emotional stressors.
13. Rhodiola Contains Compounds that support fertility in both men and women:
Rhodiola rosea supports fertility in both men and women by improving hormone levels, reducing stress, and enhancing sperm quality.
Here's a more detailed look at Rhodiola rosea and its potential role in fertility:
Potential Benefits for Fertility:
Stress Reduction:
Rhodiola is known as an adaptogen, meaning it helps the body adapt to stress, which can be a significant factor in fertility issues.
Hormone Regulation:
Some studies suggest that Rhodiola help regulate hormone levels, which are crucial for reproductive health.
Sperm Quality:
Studies suggest that Rhodiola has antioxidant properties that protect sperm from damage and improve sperm quality.
Premature Ovarian Failure:
As noted above, a study showed that Rhodiola may help women with premature ovarian failure regain their periods and achieve pregnancy.
Anti-inflammatory effects:
Rhodiola has anti-inflammatory properties which is beneficial in treating stress-induced infertility and pathologies affecting reproductive processes
Adaptogenic effect:
Rhodiola rosea is a valuable medicinal plant known mainly as an adaptogen, increasing resistance to harmful effects of various stressors
Antioxidant effects:
Rhodiola rosea extract has antioxidant and anti-inflammatory effects
How it works:
Antioxidant Activity:
Rhodiola contains compounds like salidroside and rosavins, which are antioxidants that help protect reproductive cells from damage caused by free radicals.
Hormonal Modulation:
Rhodiola may affect the hypothalamic-pituitary-gonadal (HPG) axis, which regulates hormone production, potentially leading to improved fertility.
Improved Sperm Motility:
Studies have shown that Rhodiola can improve sperm motility, mitochondrial activity, and acrosomal integrity, all of which are important for successful fertilization.
14. Rhodiola Contains Compounds that can help prevent altitude sickness:
the Tibetan climbers have used this plant for preventing altitude sickness as it reduces the symptoms of acute mountain sickness (AMS). It enhances oxygen utilization and improve the body's ability to cope with hypoxia.
Rhodiola helps with altitude sickness via:
Reduced Oxygen Consumption and Enhanced Oxygen Utilization: Studies suggest that Rhodiola rosea helps reduce oxygen consumption and improve oxygen carrying capacity, which could be beneficial at high altitudes (or if you are just working hard and have breathing issues).
Influence on Hypoxia-Related Pathways: Research suggests that Rhodiola rosea influences the hypoxia-inducible factor (HIF-1) pathway, which plays a crucial role in the body's response to low oxygen levels.
Cardiopulmonary and Nervous System Effects: Rhodiola rosea influences levels and activity of monoamines and opioid peptides, affecting cardiovascular and nervous system function, further enhancing the body's ability to cope with the effects of altitude.
History and Cultural Relevance of Rhodiola/ Goldenroot:
Known as the 'golden root' among its many names, the Rhodiola rosea herb has a bountiful history that spans across numerous global cultures and traditional uses. With its vast history of use throughout Greece, Russia, Eastern Europe, Asia, France, Iceland, Canada and Sweden, this 'super herb' comes with an intriguing and rather expansive story.
Dioscorides The "Father of Pharmacognosy" & De Materia Medica:
The first recorded use of Rhodiola rosea traces back to the Greek physician and botanist Dioscorides, who was known as the "father of pharmacognosy" and authored a highly-cherished ancient work entitled De materia medica that was widely read for more than 1,500 years. This masterwork included references to Rhodiola rosea and its medicinal applications around 77 C.E. according to researchers their work Rhodiola rosea: A Phytomedicinal Overview. Study authors Richard P. Brown, Patricia L. Gerbarg, and Zakir Ramazanov write:
"The Greek physician, Dioscorides, first recorded medicinal applications of rodia riza in 77 C.E. in De Materia Medica.3 Linnaeus renamed it Rhodiola rosea, referring to the rose-like attar (fragrance) of the fresh cut rootstock.4 For centuries, R. rosea has been used in the traditional medicine of Russia, Scandinavia, and other countries. Between 1725 and 1960, various medicinal applications of R. rosea appeared in the scientific literature of Sweden, Norway, France, Germany, the Soviet Union, and Iceland."
Used in Norway, Rhodiola rosea has been used as a cure for scurvy in cattle, to flavour beer and used as a topical preparation to promote healthy hair growth and as a treatment for dandruff.
It has long been a tradition in many places in Europe to plant house leeks (Sempervivum) and other succulent plants on roofs. Charlemagne (742–814) ordered that the roofs of his kingdom were to be planted with Rhodiola rosea and Sempervivum to protect against fire, and the custom spread across Europe. In Norway, it is known for example from the region of Telemark.
A story connects a healing plant that grew on the roofs of Bergen with Inga of Varteig (c. 1185–1234) suffering “jernbyrd” (literally “to bear the burden of iron”, a form of trial by ordeal) in 1218. She had given birth to a son named Hakon who she claimed was the son of King Håkon Sverresson of Norway. The boy was recognized as king's son by the reigning king, Inge Bårdsson, and when he died in 1217, Håkon was made king of the ‘Birkebeiner’ in Trondheim. Inga was challenged to bear the burden of iron to prove the boy's paternity, but this was only carried out the following year, at the Church of Christ in Bergen. Then Håkon and his rival for Norway's throne, Earl Skule Bårdsson - half-brother of the late King Inge - gathered with the archbishop and several chieftains. A knowledgeable man in the Earl's entourage by the name of Sigar contacted Dagfinn the farmer who was Ingas trusted man and declared himself a great friend of the King. He told that he knew a herb, the juice of which Inga could rub her hands with, thus avoiding being damaged by the hot iron.
Dagfinn was pleasantly surprised at first and asked what plant this was. Sigar replied that it grew on the roof of his house and on every man's roof in Bergen. As the story relates, Dagfinn then became suspicious and rejected both Sigar and his advice. Therefore, it does not appear that Inga used this herb, but the story testifies to a plant on the rooftops in the city with a reputation for healing burns. Since Sempervivum does not thrive here in the west, it was most likely rose root (aka Rhodiola rosea) that was planted on the peat roofs to protect against fire. She walked the proscribed nine steps, carrying iron that had been in boiling water, and after three days, the bandages were removed revealing that her hands were without burns. Thus, it was proclaimed that God himself had given a sign that she was speaking the truth. Håkon Håkonsson became king of most of Norway, with capital in Bergen, while Skule Bårdsson ruled a further part of the country with its seat in Trondheim - in constant conflict with its rival in Bergen.


In Ayurvedic medicine it is used to provide strength and support to the brain, nerves and muscles. It is often used in combination with other medicinal herbs as a therapy for anxiety and mood disorders.
Rhodiola (aka Hong Jing Tian in TCM) has long been used in Traditional Chinese Medicine to help Build Qi and Move Blood. It helps improve digestion, build stamina, and accelerate recovery from fatigue. Now known as a powerful adaptogen, rhodiola has been used by the Chinese for centuries. The ancient Greeks, and the Vikings used it to build strength.
University of Texas at El Paso summarizes on 'how Rhodiola is used' by saying:
"The medicinal raw material (rhizome with roots) is employed in traditional European folk medicine, principally in Scandinavia and the Russian Federation (Radomska-Leśniewska et al., 2015). The tender shoots and leaves are eaten in China. The plant is considered emollient and vulnerary (Quattrocchi, 2012). Rhodiola is considered astringent and bitter, but with a cool potency by Mongolian traditional medicine."
Rhodiola grows prolifically along the coast of Labrador (Canada), including rocky shorelines, sandy beaches, and up to the high tidal zone. Nunatsiavut Inuit in these remote areas have traditionally used rhodiola as food and medicine.
The Vikings, who settled in areas like Caithness and northern Scotland, harvested Rhodiola rosea from rocky cliffs and used the root to make a tea for energy and strength.
Rhodiola rosea was included in the first edition of the Pharmacopeia of Sweden in 1755.
(For a perspective from the Yukon, I will share the following pages are from an excellent book called “The Boreal Herbal: Wild Food and Medicine Plants of the North” By Herbalist, Beverley Gray. You can buy a copy of the book here.)




In Russian ethnobotany, Rhodiola rosea (also known as roseroot or golden root) has a long history of use as an adaptogen to enhance physical and mental performance, treat fatigue, and improve tolerance to stress and high altitudes. It was used to treat a variety of ailments, including infections, nervous system disorders, and impotence.
In Siberia, many people believe that drinking rhodiola rosea tea will help people live long lives. Traditionally, newlyweds are given the herb in hopes that it will boost their fertility levels as well as encourage the birth of healthy babies. In fact, some Siberian families kept the location of their rhodiola rosea crops a secret. Never disclosing the secrets to harvesting a good crop, they would trade the herb for honey, wine, and fruit.
The former Soviet Union spent decades secretly searching for energy-enhancing plants that would help their Olympians, as well as their soldiers and astronauts, perform better. The Soviets were looking for what they called “adaptogens”—plant species that would encourage the body to adapt to physical and mental stress without major side effects.
The government took these experiments so seriously that the scientists involved have been banned from speaking of their results or publishing their findings outside the country, according to Patricia Gerbarg, an Assistant Clinical Professor in Psychiatry at New York Medical College and co-author of The Rhodiola Revolution, published in 2004. In the course of writing her book, Gerbarg worked closely with the now-deceased Zakir Ramazanov, a Russian researcher who left the USSR after the Iron Curtain fell, taking confidential documents on the adaptogen tests with him to the United States.
Many of the adaptogen tests were conducted in the 1970s by the Ministry of Defense from a sealed research city in the frigid latitudes of Siberia. The USSR wanted plants that would help soldiers endure nights of frostbite and high elevations in Afghanistan. They started by testing cadets on a formula of Eleuthro (also known as Siberian Ginseng), Schisandra berries and Rose Root or Rhodiola rosea.
It was fermented before being eaten by the N. American Indigenous peoples [5].
In the rugged landscapes of Scotland, a remarkable plant thrives, symbolising the resilience of nature and the interconnectedness of our world. Rose root, also known as "Lus na laoch" in Irish, is a perennial herb with vibrant yellow blooms and holds a rich history of traditional use among the Gaelic people as well.
Functions In The Wilderness and in the Food Forest:
Ecological Functions
Habitat Provision:
Rhodiola rosea thrives in cool, temperate, and sub-arctic areas, providing habitat for a variety of wildlife, including insects, birds, and small mammals.
Food Source:
The plant's roots and leaves are a food source for certain herbivores, contributing to the local food web.
Symbiotic Relationships:
Rhodiola rosea is known to have many symbionts and plant-associated (Root Endophytic) bacteria that can protect plants from pathogens and benefit plant growth.
Rhizome Bacteria and Nitrogen Fixation:
Studies have identified bacteria associated with the rhizomes of Rhodiola rosea that enable enhanced rates of nitrogen fixation.
These bacteria include genera like Pseudomonads and Bacilli, which are known for their plant growth-promoting abilities.

Rhodiola rosea L. in Kazakhstan, Altai mountains Source: https://www.mdpi.com/1424-2818/17/1/45
Other Ecological Traits:
In addition to nitrogen fixation, some rhizome bacteria associated with Rhodiola rosea also exhibit traits related to phosphorus solubilization, siderophore production (which helps plants access iron), and indole-related compound production.
That means these plants serve the ecological function of a pioneer species that colonizes rocks along with their symbiotic bacteria partners which literally dissolve rocks via exuding mild acids, prying loose atoms and turning stone into plant food. That in turn creates biomass that decomposes into soil (allowing more complex plant communities to unfold in the future on that terrain).
Co-cultivation of selected Root Endophytic bacteria with Rhodiola species results in more than a 20% increase in root or shoot growth.
Guild Profile:
These can be grown in a shady or part sun spot in zones 4-7 and in zones 1-4 it can be grown in full sun. This species offers a fantastic opportunity to grow valuable medicine in rocky terrain if you have varying topography and soil most could consider un-usable.
You could grow a medicine garden on the rocky shore or even cliffs in a colder area.
You could grow it on the inside edge of a temperate food forest with some soil amendments, or on the outside edge of a cold climate food forest or as a central member in an arctic range regenerative medicine polyculture garden.

If one was going for maximizing productivity in a northern food forest one could include create a Pine Nut forest in zones 2-7 with mixed in cold hardy berry shrubs that like acidity, medicinal vines like Five Flavor Berries growing up the trunks of the trees, some Birch for syrup making, Larch for medicine with Rhodiola growing in the sunny patches in between the trees and hardwood mushroom logs stacked under the shade of the pines.
This plant would also make a fantastic candidate for a transition zone between an oceanic regenerative garden and a coastal food forest.

Plants with Similar Growing Conditions:
Other Rhodiola species:
Rhodiola integrifolia is another member of the Rhodiola genus that shares similar growing requirements with R. rosea.
Sedum (Stonecrop):
Like Rhodiola, Sedum species are known for their ability to tolerate cold, dry conditions and rocky soils. Several Sedum species, including Sedum telephium (Common Stonecrop), Sedum aizoon, and Sedum lineare, have been traditionally used in indigenous medicine for their medicinal properties, including wound healing, anti-inflammatory, and other effects.
Eastern Teaberry or Wild Wintergreen (Gaultheria procumbens):
Eastern teaberry is an edible wild herb with a flavor like wintergreen mints also known as wild wintergreen, checkerberry and Gaultheria procumbens. The plant contains a compound similar to aspirin, and humans have used it as a flavoring and medicine for a very long time.
Hesperis matronalis (Sweet Rocket):
This perennial is also known for its cold hardiness and ability to thrive in full sun and well-drained soil. It has various edible parts, including young leaves, flowers, and seeds, that can be used in salads or as a garnish.
Also known as Dame's Rocket, has been used traditionally for its medicinal properties, including being antiscorbutic, diaphoretic, and diuretic, with the leaves being best harvested when the plant is in flower.
Astragalus species (Milkvetch):
Some Astragalus species are adapted to harsh climates and can tolerate drought and cold temperatures. This plant has a long history of use in traditional Chinese medicine, often used as a tonic to boost energy, stamina, and vitality, and is promoted for its potential immune-boosting and anti-inflammatory properties.
Lewisia rediviva
Lewisia species are native to North America and are adapted to cold mountainous regions. Lewisia rediviva (Bitterroot), has a history of medicinal uses among indigenous cultures, with the root being used for sore throats, promoting milk flow during lactation, and potentially as a blood purifier and for heart pain.
Commonly referred to as Adam’s needle, Yucca filamentosa is a hardy yucca species that can withstand cold temperatures and snow. Has a long history of traditional medicinal uses, particularly for skin conditions, joint pain, and as a soap substitute.
Sempervivum (Hens and Chicks):
These rosette-forming succulents are known for their resilience in cold climates and come in various colors and sizes. These are commonly used for ground cover.
Has a history of medicinal uses, with the juice or leaves used externally for skin conditions like burns, wounds, and insect bites, and internally for diarrhea and other ailments.
Agastache foeniculum (aka Anise Hyssop):
Great for pollinators, you can make tea from the leaves and flavor recipes with the leaves.
Opuntia humifusa (Eastern Prickly Pear Cactus):
Native to North America, this prickly pear cactus is cold-hardy and can tolerate freezing temperatures. The Eastern Prickly Pear Cactus (Opuntia humifusa) has been traditionally used for its medicinal properties, including treating diabetes, high cholesterol, obesity, and hangovers, and also has antiviral and anti-inflammatory effects.
Could be grown along side this plant on the edge of temperate food forests.
Larch trees, particularly Siberian and American (Tamarack) larches:
These trees are known for their cold hardiness and ability to thrive in harsh climates, often found in northern regions.
Edible parts of Tamarack: Tender young shoots can be cooked as a vegetable. And like most trees, the inner bark can be dried and ground for flour. And like most conifers, you can use the fresh needles for a tea. And like most conifers, it may be an acquired taste! A tea is made from the roots. Tamarack gum tastes like candy. The sap contains a natural sugar with a flavor like bitter honey, called galactan.
The dried, powdered gum can be used as baking powder.
Medicinal use of Tamarack: Tamarack was employed medicinally by a number of native North American indigenous tribes who used it to treat a variety of complaints. It is little used in modern herbalism. A tea made from the bark is said to be an alterative, diuretic, laxative and tonic. It is used in the treatment of jaundice, anaemia, rheumatism, colds and skin ailments. It is gargled in the treatment of sore throats and applied as a poultice to sores, swellings and burns. A tea made from the leaves is astringent. It is used in the treatment of piles, diarrhoea etc. An infusion of the buds and bark is used as an expectorant. The needles and inner bark are disinfectant and laxative. A tea is used in the treatment of coughs. A poultice made from the warm, boiled inner bark is applied to wounds to draw out infections, to burns, frostbite and deep cuts. The resin is chewed as a cure for indigestion. It has also been used in the treatment of kidney and lung disorders, and as a dressing for ulcers and burns.
Very cold hardy, powerful medicine in the foliage and bark.
Edible young cones and pollen.
Schisandra chinensis (aka "five flavor fruit"):
Cold hardy to zone three, so if you were growing roseroot on the edge of a cold climate food forest you could grow the five flavor fruit vines up the trees for support.
Also Very cold hardy, powerful medicine in the foliage and bark.
Spruce trees provide a bounty of medicine, food, habitat and essential materials for countless species and cultures globally.
Edible young cones and pollen.
Cold Hardy Pines (like Siberian and Korean Stone Pines)
Can grow in zones 1-8 and provide nutrient dense seeds (aka “pine nuts”), edible pollen, medicinal foliage, edible young cones, basket weaving materials and shade for growing mushrooms
Extremely cold hardy, leaves can be used for tea, medicine from the bark, and you can tap the trees like maples to make a nutrient dense syrup.
Great for companion planting near a Regenerative Ocean Garden that transitions into a coastal food forest:
If you live on an ocean coastline in a cold zone Rhodiola rosea can grow on the rocky cliffs, shale or hill above a regenerative ocean vegetable garden/clam garden.
Practical Uses:
How to make Rhodiola rosea tea:
Another way to benefit from rhodiola is to drink Rhodiola rosea tea, traditionally used to help calm nerves, reduce anxiety and promote restful sleep. To prepare homemade rhodiola tea, you’ll first need to purchase rhodiola roots that have been dried and ground.
Start by steeping about five grams of rhodiola roots in hot water. Either use a steeper or pack tea bags with the root. Make sure the water is not very hot or boiling, keeping it no higher than 85 degrees Fahrenheit (boiling point is 212 degrees F). For the best results, steep the tea for about four hours.
To speed up this process, you can also use rhodiola tinctures and liquid extract, which can be added to warm water with lemon or another herbal tea, such as chamomile or green tea.
Dried root: 1g TID. Standardized extract: (3% rosavin, 1% salidroside) 200-600mg QD. Low dose tends to be stimulating and high dose is more sedating.


Seed Propagation:
The seeds require a solid stratification cycle given their native habitat and I have also found to associate with symbiotic bacteria so I have experimented enriching the seed's microbiome with a diluted EM1 solution and saw good results with that method.
For additional info on the species of Endophytic bacteria that enhance germination, growth rates and nitrogen fixation: https://www.mdpi.com/2076-2607/13/1/13
For a more DIY approach, based on my study of Mosses and Lichens I have found that rhodiola rosea and her cousin like to associate with a similar spectrum of Endophytic bacteria to many ocean, alpine and artctic tundra species of lichen and moss. Thus, if you wanted to create your own microbial inoculant for starting rhodiola rosea seeds (or enriching the rhizosphere of established plants in containers) you could take a section of mossy rock/lichen and then use it as a source of indigenous microorganisms to create a sort of IMO with an adapted technique similar to this.
Seed - surface sow in a sunny position in a greenhouse in spring. Do not let the compost dry out. The seed usually germinates in 2 - 4 weeks at 10°c. Prick out the seedlings into individual pots when they are large enough to handle, and grow them on in the greenhouse for their first winter. Plant out in early summer of the following year.
Division in August to October[12]. Very easy, larger divisions can be planted out direct into their permanent positions. We have found that it is better to pot up the smaller divisions and grow them on in light shade in a cold frame until they are well established before planting them out in late spring or early summer.
Cuttings taken in the growing season[12]. Basal shoots in early summer are easiest. Harvest the shoots with plenty of underground stem when they are about 8 - 10cm above the ground. Pot them up into individual pots and keep them in light shade in a cold frame or greenhouse until they are rooting well. Plant them out in the summer.
Seed sources:
https://www.saltspringseeds.com/products/roseroot-rhodiola-rosea
https://strictlymedicinalseeds.com/product/rhodiola-rhodiola-rosea-packet-of-100-seeds/
https://ferriseeds.com/products/rhodiola-roseroot-rhodiola-rosea
Cultivation details:
Germination benefits greatly from cold conditioning/stratification, possibly ~ 6 weeks at 5 Celsius or colder, though typically seed is sown on moist sterilized potting soil (in plug trays – 72 cell trays are popular) during the winter and placed outside for two months or more, preferably with snow cover. Seeds can be covered lightly or pressed into the soil surface, but should not be buried too deeply or allowed to dry out completely. In Canada, rhodiola sprouts appear in late April or early May, after daytime temperatures increase, and can withstand significant frosts. Alternatively, strategies which utilize or mimic ethylene gas may also promote germination.
Young rhodiola seedlings grow slowly, and do better in a location semi-sheltered from sun and wind. They grow slowly, suffering both when the soil remains saturated with moisture for extended periods and when the soil becomes very dry. Thus a balance between overwatering and drought conditions should be maintained. Mild fertilization may be beneficial, but is not required. After a month or two, when a stalk is sent up from the rosette of seed leaves, seedlings can be exposed to more sun to maximize growth. Seedlings can be transplanted in their first year, but can also be kept in plug trays for a year or two to minimize weeding in the field. Excellent transplant survival rates can be achieved any time the ground is not frozen, even with dormant (leafless) plugs in the fall. Eventually plant growth will suffer if seedlings are not planted out.
Ideal growing site components include full or almost full sun, good drainage during the spring runoff and some shelter from the wind. While the latter is not imperative, it will help conserve soil moisture and enhance growth. R. rosea is very drought tolerant and does not require irrigation, however, it will benefit from regular watering – natural or otherwise. Field spacing depends on the chosen weed control system, especially if plastic mulch is used. One foot in-row spacing, with eight-inch between-row spacing of plants is an average for current trials, giving three to four rows of plants per (mulched) bed. Path spacing between beds will vary with the weeding regime, or a solid (pathless) planting may be preferred.
Time to harvest can be as short as three growing seasons, when roots can attain 0.75% rosavin content or better, though four to five year’s growth will provide greater root biomass and a rosavin content of 1% or more. The roots tend to deteriorate from within as they age, harboring patches of necrotic tissue (or “heart-rot”) to which they will eventually succumb. The upshot is that – while there may be 75-year old plants in the wild – the maximum age of a commercial field may only be six or seven years. The dynamics of root attrition due to disease are not yet understood, and may differ with various cultivars and soil conditions. Initial indications are that fertilization is not beneficial under normal conditions.
R. rosea is an adaptable species, and as such appears to do well in a variety of soil types, from rocky gravel through heavy clay to silty, sandy and peaty loam soil types. The relationship between soil pH and rosavin levels is presently poorly understood, but may favor acidity. As a circumpolar species, Rhodiola does well at high latitudes, where its production of rosavins assists survival under harsh conditions. How it performs in warmer climates will be an interesting experiment. (source)
For more info on growing:
Recipes:
Rhodiola root powder can be added to smoothies, yogurts, or baked goods. Add a teaspoon of powder to your favorite recipes to enjoy the benefits of this adaptogenic plant in your daily diet.
The young, succulent leaves and shoots of Rhodiola rosea (roseroot) are edible, though have a slightly bitter taste and are best eaten raw or cooked like spinach, and are sometimes added to salads along side other sweet and savory ingredients.
I like to make Miso paste infused with the Rhodiola root powder and also use sections of the dried roots along side Kombu and shiitake stems for building a medicinally potent and flavorful soup broth base.
In Russia, rhodiola is used in different ways: jam, herbal tea, elixir, fruit paste. In Eastern Europe you can easily find syrup made from rhodiola rosea.
Rhodiola rosea can be found in the form of powder, dried roots, used in decoction, maceration, extract and tincture.
Immune Supporting Soup Broth with Reishi, Rhodiola and Astragalus:

Warm Cacao Latte With Rhodiola
Beet, Protein and Rhodiola Smoothie:
Adaptogenic Cacao Rhodiola and Ashwagandha Granola:
Rose Pudding with Cardamom Syrup and Rhodiola & Pistachio Crumble:
Here are some of my own recipes that I think would be lovely with the addition of a teaspoon or two of Rhodiola (Golden Root) powder to give them an extra apoptogenic kick:
7 Fold Flame Sriracha
This is Installment #12 of the (Stacking Functions in the Garden, Food Forest and Medicine Cabinet : The Regenerative Way From Seed To Apothecary series.
Adaptogenic Super Taco Mix
I love Mexican food (well I love a lot of food with cultural roots south of the US border going all the way down to traditional Incan territory really) so I have been experimenting with combining my passion for making tacos, burritos, enchiladas, fajitas and heuvos rancheros with my more recently acquired knowledge of
Vegetarian Khao Poon
I have decided I will be sharing one recipe from my book (Recipes For Reciprocity: The Regenerative Way From Seed To Table) per week for the next month to give all my kind and generous Substack subscribers a sneak peak and taste of what is in my book (which was
Golden Dragon Chi
This is Installment #10 of the (Stacking Functions in the Garden, Food Forest and Medicine Cabinet : The Regenerative Way From Seed To Apothecary series.
Five Bean Spring Chili with Ramps, Nettles and Sautéed Morels
I recently used some spring harvests of Ramps, Nettles, Garlic Mustard, Goji leaves/shoots, Morels and combined it with dried beans, frozen garden tomatoes as well as Goji Berries, freeze dried garden peppers, some fermented fire roasted hot peppers and dried spices/herbs from our 2023 harvests (with some wild rice and
Garden Minestrone Soup
I have always loved minestrone soup over since my childhood as my mom was a great soup maker. When her homemade version was not available I would go to the grocery store to try and find a facsimile but it was never the same. Thus, minestrone was one of the very first soups I taught myself to make in my youth and I have been improving on my recipe ever s…
For more ideas on ADAPTOGENS + FOOD PAIRINGS:
References:
https://www.mdpi.com/1422-0067/24/15/12293
https://greenmedinfo.com/substance/rhodiola-tibetan-ginseng
https://pmc.ncbi.nlm.nih.gov/articles/PMC9351785/
https://practicalplants.org/wiki/rhodiola_rosea/#:~:text=Cultivation.%20Prefers%20a%20fertile%20well%20drained%20open,damp%20conditions%20but%20prefers%20a%20raised%20well%2Ddrained
https://pubmed.ncbi.nlm.nih.gov/37641937/
https://www.wilderlandbotanicals.com/pages/regenerative-organic-agriculture?srsltid=AfmBOoq7LtYxQcSzNhKYVp7AnQbU-suOucgaAurLJIQ2yteYol1oeSLl
https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2016.00254/full
www.tellurideinstitute.org/wp-content/uploads/2018/04/brittneymanzagol_final_paper.pdf
https://abs.pensoft.net/article/78936/
https://www.mdpi.com/2223-7747/12/6/1364
https://www.sciencedirect.com/science/article/pii/S1021949815000563
https://pmc.ncbi.nlm.nih.gov/articles/PMC7435400/
https://beverleygray.com/2016/03/16/irish-cures-tradition/
https://www.learningherbs.com/blog/rhodiola-uses#gsc.tab=0
https://pmc.ncbi.nlm.nih.gov/articles/PMC8898776/
https://pmc.ncbi.nlm.nih.gov/articles/PMC4590898/
https://www.sciencedirect.com/science/article/abs/pii/S0014299920304866
https://as-botanicalstudies.springeropen.com/articles/10.1186/s40529-021-00327-4
https://thenaturopathicherbalist.com/2015/09/25/rhodiola-rosea/
https://www.healthline.com/nutrition/rhodiola-rosea#managing-diabetes
https://pmc.ncbi.nlm.nih.gov/articles/PMC4590898/
https://www.sciencedirect.com/science/article/pii/S1878535223010328
https://versicolor.ca/nswfsOLDsite/species/Crassulaceae/SedRosea/species.html
http://naeb.brit.org/uses/32829/
https://www.researchgate.net/publication/226195024_Specific_Characteristics_of_Rhodiola_rosea_Growth_and_Development_under_the_Photoculture_Conditions
https://gobotany.nativeplanttrust.org/species/rhodiola/rosea/
https://www.mhprofessional.com/9780071464734-usa-womens-encyclopedia-of-natural-medicine-group ↩︎
https://philipcarr-gomm.com/essay/working-sacred-plants-druid-tradition/
Ishaque, S.; Shamseer, L.; Bukutu, C.; Vohra, S. Rhodiola rosea for physical and mental fatigue: A systematic review. BMC Complement. Altern. Med. 2012, 12, 70. [Google Scholar] [CrossRef] [Green Version]
Polumackanycz, M.; Konieczynski, P.; Orhan, I.E.; Abaci, N.; Viapiana, A. Chemical Composition, Antioxidant and Anti-Enzymatic Activity of Golden Root (Rhodiola rosea L.) Commercial Samples. Antioxidants 2022, 11, 919. [Google Scholar] [CrossRef] [PubMed]
Panossian, A.; Wikman, G.; Sarris, J. Rosenroot (Rhodiola rosea): Traditional use, chemical composition, pharmacology and clinical efficacy. Phytomed. Int. J. Phytother. Phytopharm. 2010, 17, 481–493. [Google Scholar] [CrossRef]
Tao, H.; Wu, X.; Cao, J.; Peng, Y.; Wang, A.; Pei, J.; Xiao, J.; Wang, S.; Wang, Y. Rhodiola species: A comprehensive review of traditional use, phytochemistry, pharmacology, toxicity, and clinical study. Med. Res. Rev. 2019, 39, 1779–1850. [Google Scholar] [CrossRef] [PubMed]
Chiang, H.M.; Chen, H.C.; Wu, C.S.; Wu, P.Y.; Wen, K.C. Rhodiola plants: Chemistry and biological activity. J. Food Drug Anal. 2015, 23, 359–369. [Google Scholar] [CrossRef] [PubMed]
Kelly, G.S. Rhodiola rosea: A possible plant adaptogen. Altern. Med. Rev. J. Clin. Ther. 2001, 6, 293–302. [Google Scholar]
Kucinskaite, A.; Briedis, V.; Savickas, A. Experimental analysis of therapeutic properties of Rhodiola rosea L. and its possible application in medicine. Medicina 2004, 40, 614–619. [Google Scholar]
De Bock, K.; Eijnde, B.O.; Ramaekers, M.; Hespel, P. Acute Rhodiola rosea intake can improve endurance exercise performance. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 298–307. [Google Scholar] [CrossRef] [Green Version]
Carlini, E.A. Plants and the central nervous system. Pharmacol. Biochem. Behav. 2003, 75, 501–512. [Google Scholar] [CrossRef]
Tolonen, A.; Pakonen, M.; Hohtola, A.; Jalonen, J. Phenylpropanoid glycosides from Rhodiola rosea. Chem. Pharm. Bull. 2003, 51, 467–470. [Google Scholar] [CrossRef] [Green Version]
Ivanova Stojcheva, E.; Quintela, J.C. The Effectiveness of Rhodiola rosea L. Preparations in Alleviating Various Aspects of Life-Stress Symptoms and Stress-Induced Conditions-Encouraging Clinical Evidence. Molecules 2022, 27, 3902. [Google Scholar] [CrossRef] [PubMed]
Döring, K.; Langeder, J.; Duwe, S.; Tahir, A.; Grienke, U.; Rollinger, J.M.; Schmidtke, M. Insights into the direct anti-influenza virus mode of action of Rhodiola rosea. Phytomed. Int. J. Phytother. Phytopharm. 2022, 96, 153895. [Google Scholar] [CrossRef] [PubMed]
Panossian, A.; Brendler, T. The Role of Adaptogens in Prophylaxis and Treatment of Viral Respiratory Infections. Pharmaceuticals 2020, 13, 236. [Google Scholar] [CrossRef]
Wagner, H.; Nörr, H.; Winterhoff, H. Plant adaptogens. Phytomed. Int. J. Phytother. Phytopharm. 1994, 1, 63–76. [Google Scholar] [CrossRef]
Khanna, K.; Mishra, K.P.; Ganju, L.; Singh, S.B. Golden root: A wholesome treat of immunity. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 87, 496–502. [Google Scholar] [CrossRef] [PubMed]
Dement’eva, L.A.; Iaremenko, K.V. Effect of a Rhodiola extract on the tumor process in an experiment. Vopr. Onkol. 1987, 33, 57–60. [Google Scholar]
Udintsev, S.N.; Schakhov, V.P. Decrease of cyclophosphamide haematotoxicity by Rhodiola rosea root extract in mice with Ehrlich and Lewis transplantable tumors. Eur. J. Cancer 1991, 27, 1182. [Google Scholar] [CrossRef]
Li, Y.; Pham, V.; Bui, M.; Song, L.; Wu, C.; Walia, A.; Uchio, E.; Smith-Liu, F.; Zi, X. Rhodiola rosea L.: An herb with anti-stress, anti-aging, and immunostimulating properties for cancer chemoprevention. Curr. Pharmacol. Rep. 2017, 3, 384–395. [Google Scholar] [CrossRef]
Recio, M.C.; Giner, R.M.; Máñez, S. Immunmodulatory and Antiproliferative Properties of Rhodiola Species. Planta Med. 2016, 82, 952–960. [Google Scholar] [CrossRef]
Kim, S.H.; Hyun, S.H.; Choung, S.Y. Antioxidative effects of Cinnamomi cassiae and Rhodiola rosea extracts in liver of diabetic mice. BioFactors 2006, 26, 209–219. [Google Scholar] [CrossRef]
Tang, C.; Zhao, C.C.; Yi, H.; Geng, Z.J.; Wu, X.Y.; Zhang, Y.; Liu, Y.; Fan, G. Traditional Tibetan Medicine in Cancer Therapy by Targeting Apoptosis Pathways. Front. Pharm. 2020, 11, 976. [Google Scholar] [CrossRef] [PubMed]
Malík, M.; Tlustoš, P. Nootropic Herbs, Shrubs, and Trees as Potential Cognitive Enhancers. Plants 2023, 12, 1364. [Google Scholar] [CrossRef] [PubMed]
Esmaealzadeh, N.; Iranpanah, A.; Sarris, J.; Rahimi, R. A literature review of the studies concerning selected plant-derived adaptogens and their general function in body with a focus on animal studies. Phytomed. Int. J. Phytother. Phytopharm. 2022, 105, 154354. [Google Scholar] [CrossRef]
Arbuzov, A.G.; Maslov, L.N.; Burkova, V.N.; Krylatov, A.V.; Konkovskaia Iu, N.; Safronov, S.M. Phytoadaptogens-induced phenomenon similar to ischemic preconditioning. Ross. Fiziol. Zhurnal I. M. Sechenova 2009, 95, 398–404. [Google Scholar]
Maĭmeskulova, L.A.; Maslov, L.N. Anti-arrhythmic effect of phytoadaptogens. Eksp. Klin. Farm. 2000, 63, 29–31. [Google Scholar]
Maslov, L.N.; Lishmanov, Y.B.; Arbuzov, A.G.; Krylatov, A.V.; Budankova, E.V.; Konkovskaya, Y.N.; Burkova, V.N.; Severova, E.A. Antiarrhythmic activity of phytoadaptogens in short-term ischemia-reperfusion of the heart and postinfarction cardiosclerosis. Bull. Exp. Biol. Med. 2009, 147, 331–334. [Google Scholar] [CrossRef]
Maslov, L.N.; Lishmanov Iu, B. Cardioprotective and antiarrhythmic properties of Rhodiolae roseae preparations. Eksp. Klin. Farm. 2007, 70, 59–67. [Google Scholar]
Maslova, L.V.; Kondrat’ev, B.; Maslov, L.N.; Lishmanov Iu, B. The cardioprotective and antiadrenergic activity of an extract of Rhodiola rosea in stress. Eksp. Klin. Farm. 1994, 57, 61–63. [Google Scholar]
Liu, Y.; Weng, W.; Gao, R.; Liu, Y. New Insights for Cellular and Molecular Mechanisms of Aging and Aging-Related Diseases: Herbal Medicine as Potential Therapeutic Approach. Oxid. Med. Cell. Longev. 2019, 2019, 4598167. [Google Scholar] [CrossRef] [Green Version]
Olennikov, D.N.; Chirikova, N.K.; Vasilieva, A.G.; Fedorov, I.A. LC-MS Profile, Gastrointestinal and Gut Microbiota Stability and Antioxidant Activity of Rhodiola rosea Herb Metabolites: A Comparative Study with Subterranean Organs. Antioxidants 2020, 9, 526. [Google Scholar] [CrossRef]
Li, Y.; Wu, J.; Shi, R.; Li, N.; Xu, Z.; Sun, M. Antioxidative Effects of Rhodiola Genus: Phytochemistry and Pharmacological Mechanisms against the Diseases. Curr. Top. Med. Chem. 2017, 17, 1692–1708. [Google Scholar] [CrossRef] [PubMed]
Pu, W.L.; Zhang, M.Y.; Bai, R.Y.; Sun, L.K.; Li, W.H.; Yu, Y.L.; Zhang, Y.; Song, L.; Wang, Z.X.; Peng, Y.F.; et al. Anti-inflammatory effects of Rhodiola rosea L.: A review. Biomed. Pharm. Biomed. Pharm. 2020, 121, 109552. [Google Scholar] [CrossRef] [PubMed]
Anghelescu, I.G.; Edwards, D.; Seifritz, E.; Kasper, S. Stress management and the role of Rhodiola rosea: A review. Int. J. Psychiatry Clin. Pract. 2018, 22, 242–252. [Google Scholar] [CrossRef] [Green Version]
Sun, S.; Tuo, Q.; Li, D.; Wang, X.; Li, X.; Zhang, Y.; Zhao, G.; Lin, F. Antioxidant Effects of Salidroside in the Cardiovascular System. Evid. Based Complement Altern. Med. 2020, 2020, 9568647. [Google Scholar] [CrossRef] [PubMed]
Zhang, X.; Xie, L.; Long, J.; Xie, Q.; Zheng, Y.; Liu, K.; Li, X. Salidroside: A review of its recent advances in synthetic pathways and pharmacological properties. Chem.-Biol. Interact. 2021, 339, 109268. [Google Scholar] [CrossRef]
Sun, A.Q.; Ju, X.L. Advances in Research on Anticancer Properties of Salidroside. Chin. J. Integr. Med. 2021, 27, 153–160. [Google Scholar] [CrossRef]
Zhao, C.C.; Wu, X.Y.; Yi, H.; Chen, R.; Fan, G. The Therapeutic Effects and Mechanisms of Salidroside on Cardiovascular and Metabolic Diseases: An Updated Review. Chem. Biodivers. 2021, 18, e2100033. [Google Scholar] [CrossRef]
Han, J.; Luo, L.; Wang, Y.; Wu, S.; Kasim, V. Therapeutic potential and molecular mechanisms of salidroside in ischemic diseases. Front. Pharm. 2022, 13, 974775. [Google Scholar] [CrossRef]
Li, Y.; Cai, M.; Mao, G.X.; Shu, Q.F.; Liu, X.B.; Liu, X.L. Preclinical Evidence and Possible Mechanisms of Rhodiola rosea L. and Its Components for Ischemic Stroke: A Systematic Review and Meta-Analysis. Front. Pharm. 2021, 12, 736198. [Google Scholar] [CrossRef]
Fan, F.; Yang, L.; Li, R.; Zou, X.; Li, N.; Meng, X.; Zhang, Y.; Wang, X. Salidroside as a potential neuroprotective agent for ischemic stroke: A review of sources, pharmacokinetics, mechanism and safety. Biomed. Pharm. Biomed. Pharm. 2020, 129, 110458. [Google Scholar] [CrossRef]
Jin, M.; Wang, C.; Xu, Y.; Zhang, Z.; Wu, X.; Ye, R.; Zhang, Q.; Han, D. Pharmacological effects of salidroside on central nervous system diseases. Biomed. Pharm. Biomed. Pharm. 2022, 156, 113746. [Google Scholar] [CrossRef]
Rohloff, J. Volatiles from rhizomes of Rhodiola rosea L. Phytochemistry 2002, 59, 655–661. [Google Scholar] [CrossRef]
Akgul, Y.; Ferreira, D.; Abourashed, E.A.; Khan, I.A. Lotaustralin from Rhodiola rosea roots. Fitoterapia 2004, 75, 612–614. [Google Scholar] [CrossRef]
Ali, Z.; Fronczek, F.R.; Khan, I.A. Phenylalkanoids and monoterpene analogues from the roots of Rhodiola rosea. Planta Med. 2008, 74, 178–181. [Google Scholar] [CrossRef] [PubMed]
Yousef, G.G.; Grace, M.H.; Cheng, D.M.; Belolipov, I.V.; Raskin, I.; Lila, M.A. Comparative phytochemical characterization of three Rhodiola species. Phytochemistry 2006, 67, 2380–2391. [Google Scholar] [CrossRef] [PubMed]
Petsalo, A.; Jalonen, J.; Tolonen, A. Identification of flavonoids of Rhodiola rosea by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2006, 1112, 224–231. [Google Scholar] [CrossRef] [PubMed]
Chen, T.S.; Liou, S.Y.; Chang, Y.L. Antioxidant evaluation of three adaptogen extracts. Am. J. Chin. Med. 2008, 36, 1209–1217. [Google Scholar] [CrossRef]
Ma, G.; Li, W.; Dou, D.; Chang, X.; Bai, H.; Satou, T.; Li, J.; Sun, D.; Kang, T.; Nikaido, T.; et al. Rhodiolosides A-E, monoterpene glycosides from Rhodiola rosea. Chem. Pharm. Bull. 2006, 54, 1229–1233. [Google Scholar] [CrossRef] [Green Version]
Mirmazloum, I.; Ladányi, M.; György, Z. Changes in the Content of the Glycosides, Aglycons and their Possible Precursors of Rhodiola rosea during the Vegetation Period. Nat. Prod. Commun. 2015, 10, 1413–1416. [Google Scholar] [CrossRef] [Green Version]
Guo, N.; Zhu, M.; Han, X.; Sui, D.; Wang, Y.; Yang, Q. The metabolism of salidroside to its aglycone p-tyrosol in rats following the administration of salidroside. PLoS ONE 2014, 9, e103648. [Google Scholar] [CrossRef]
Zhang, Y.; Li, L.; Lin, L.; Liu, J.; Zhang, Z.; Xu, D.; Xiang, F. Pharmacokinetics, tissue distribution, and excretion of salidroside in rats. Planta Med. 2013, 79, 1429–1433. [Google Scholar] [CrossRef]
Panossian, A. Challenges in phytotherapy research. Front. Pharmacol. 2023, 14, 1199516. [Google Scholar] [CrossRef] [PubMed]
Shen, B.; Truong, J.; Helliwell, R.; Govindaraghavan, S.; Sucher, N.J. An in vitro study of neuroprotective properties of traditional Chinese herbal medicines thought to promote healthy ageing and longevity. BMC Complement. Altern. Med. 2013, 13, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Edwards, D.; Heufelder, A.; Zimmermann, A. Therapeutic effects and safety of Rhodiola rosea extract WS® 1375 in subjects with life-stress symptoms--results of an open-label study. Phytother. Res. PTR 2012, 26, 1220–1225. [Google Scholar] [CrossRef]
Zhang, S.; Deng, N.; Zheng, B.; Li, T.; Liu, R.H. The effect of in vitro gastrointestinal digestion on the phenolic profiles, bioactivities and bioaccessibility of Rhodiola. Food Funct. 2022, 13, 5752–5765. [Google Scholar] [CrossRef]
Grover, A.; Joshi, A. An overview of chronic disease models: A systematic literature review. Glob. J. Health Sci. 2014, 7, 210–227. [Google Scholar] [CrossRef] [Green Version]
Reynolds, R.; Dennis, S.; Hasan, I.; Slewa, J.; Chen, W.; Tian, D.; Bobba, S.; Zwar, N. A systematic review of chronic disease management interventions in primary care. BMC Fam. Pract. 2018, 19, 11. [Google Scholar] [CrossRef]
Nabavi, S.F.; Braidy, N.; Orhan, I.E.; Badiee, A.; Daglia, M.; Nabavi, S.M. Rhodiola rosea L. and Alzheimer’s Disease: From Farm to Pharmacy. Phytother. Res. PTR 2016, 30, 532–539. [Google Scholar] [CrossRef] [PubMed]
Majewska, A.; Hoser, G.; Furmanowa, M.; Urbańska, N.; Pietrosiuk, A.; Zobel, A.; Kuraś, M. Antiproliferative and antimitotic effect, S phase accumulation and induction of apoptosis and necrosis after treatment of extract from Rhodiola rosea rhizomes on HL-60 cells. J. Ethnopharmacol. 2006, 103, 43–52. [Google Scholar] [CrossRef]
Bai, X.L.; Deng, X.L.; Wu, G.J.; Li, W.J.; Jin, S. Rhodiola and salidroside in the treatment of metabolic disorders. Mini Rev. Med. Chem. 2019, 19, 1611–1626. [Google Scholar] [CrossRef]
Maĭmeskulova, L.A.; Maslov, L.N.; Lishmanov Iu, B.; Krasnov, E.A. The participation of the mu-, delta- and kappa-opioid receptors in the realization of the anti-arrhythmia effect of Rhodiola rosea. Eksp. Klin. Farm. 1997, 60, 38–39. [Google Scholar]
Morgan, L.A.; Grundmann, O. Preclinical and Potential Applications of Common Western Herbal Supplements as Complementary Treatment in Parkinson’s Disease. J. Diet Suppl. 2017, 14, 453–466. [Google Scholar] [CrossRef] [PubMed]
Van Diermen, D.; Marston, A.; Bravo, J.; Reist, M.; Carrupt, P.A.; Hostettmann, K. Monoamine oxidase inhibition by Rhodiola rosea L. roots. J. Ethnopharm. 2009, 122, 397–401. [Google Scholar] [CrossRef] [PubMed]
Magani, S.K.J.; Mupparthi, S.D.; Gollapalli, B.P.; Shukla, D.; Tiwari, A.K.; Gorantala, J.; Yarla, N.S.; Tantravahi, S. Salidroside—Can it be a Multifunctional Drug? Curr. Drug Metab. 2020, 21, 512–524. [Google Scholar] [CrossRef]
Li, T.; Feng, Y.; Yang, R.; Wu, L.; Li, R.; Huang, L.; Yang, Q.; Chen, J. Salidroside Promotes the Pathological α-Synuclein Clearance Through Ubiquitin-Proteasome System in SH-SY5Y Cells. Front. Pharm. 2018, 9, 377. [Google Scholar] [CrossRef] [Green Version]
Li, R.; Wang, S.; Li, T.; Wu, L.; Fang, Y.; Feng, Y.; Zhang, L.; Chen, J.; Wang, X. Salidroside Protects Dopaminergic Neurons by Preserving Complex I Activity via DJ-1/Nrf2-Mediated Antioxidant Pathway. Park. Dis. 2019, 2019, 6073496. [Google Scholar] [CrossRef] [Green Version]
Li, T.; Zhang, W.; Kang, X.; Yang, R.; Li, R.; Huang, L.; Chen, J.; Yang, Q.; Sun, X. Salidroside protects dopaminergic neurons by regulating the mitochondrial MEF2D-ND6 pathway in the MPTP/MPP(+) -induced model of Parkinson’s disease. J. Neurochem. 2020, 153, 276–289. [Google Scholar] [CrossRef]
Chen, S.; Cai, F.; Wang, J.; Yang, Z.; Gu, C.; Wang, G.; Mao, G.; Yan, J. Salidroside protects SH-SY5Y from pathogenic α-synuclein by promoting cell autophagy via mediation of mTOR/p70S6K signaling. Mol. Med. Rep. 2019, 20, 529–538. [Google Scholar] [CrossRef] [Green Version]
Zhao, H.B.; Ma, H.; Ha, X.Q.; Zheng, P.; Li, X.Y.; Zhang, M.; Dong, J.Z.; Yang, Y.S. Salidroside induces rat mesenchymal stem cells to differentiate into dopaminergic neurons. Cell Biol. Int. 2014, 38, 462–471. [Google Scholar] [CrossRef]
Wang, S.; He, H.; Chen, L.; Zhang, W.; Zhang, X.; Chen, J. Protective effects of salidroside in the MPTP/MPP(+)-induced model of Parkinson’s disease through ROS-NO-related mitochondrion pathway. Mol. Neurobiol. 2015, 51, 718–728. [Google Scholar] [CrossRef]
Zhang, B.; Wang, Y.; Li, H.; Xiong, R.; Zhao, Z.; Chu, X.; Li, Q.; Sun, S.; Chen, S. Neuroprotective effects of salidroside through PI3K/Akt pathway activation in Alzheimer’s disease models. Drug Des. Devel. Ther. 2016, 10, 1335–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Wang, H.; Li, Q.; Sun, S.; Chen, S. Neuroprotective Effects of Salidroside in a Mouse Model of Alzheimer’s Disease. Cell Mol. Neurobiol. 2020, 40, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
Taniguchi, K.; Yamamoto, F.; Arai, T.; Yang, J.; Sakai, Y.; Itoh, M.; Mamada, N.; Sekiguchi, M.; Yamada, D.; Saitoh, A.; et al. Tyrosol Reduces Amyloid-β Oligomer Neurotoxicity and Alleviates Synaptic, Oxidative, and Cognitive Disturbances in Alzheimer’s Disease Model Mice. J. Alzheimers Dis. 2019, 70, 937–952. [Google Scholar] [CrossRef]
Liao, Z.L.; Su, H.; Tan, Y.F.; Qiu, Y.J.; Zhu, J.P.; Chen, Y.; Lin, S.S.; Wu, M.H.; Mao, Y.P.; Hu, J.J.; et al. Salidroside protects PC-12 cells against amyloid β-induced apoptosis by activation of the ERK1/2 and AKT signaling pathways. Int. J. Mol. Med. 2019, 43, 1769–1777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Amsterdam, J.D.; Panossian, A.G. Rhodiola rosea L. as a putative botanical antidepressant. Phytomed. Int. J. Phytother. Phytopharm. 2016, 23, 770–783. [Google Scholar] [CrossRef] [PubMed]
Limanaqi, F.; Biagioni, F.; Busceti, C.L.; Polzella, M.; Fabrizi, C.; Fornai, F. Potential Antidepressant Effects of Scutellaria baicalensis, Hericium erinaceus and Rhodiola rosea. Antioxidants 2020, 9, 234. [Google Scholar] [CrossRef] [Green Version]
Afzal, M.; Sayyed, N.; Alharbi, K.S.; Alzarea, S.I.; Alshammari, M.S.; Alomar, F.A.; Alenezi, S.K.; Quazi, A.M.; Alzarea, A.I.; Kazmi, I. Anti-Huntington’s Effect of Rosiridin via Oxidative Stress/AchE Inhibition and Modulation of Succinate Dehydrogenase, Nitrite, and BDNF Levels against 3-Nitropropionic Acid in Rodents. Biomolecules 2022, 12, 1023. [Google Scholar] [CrossRef]
Fan, F.; Xu, N.; Sun, Y.; Li, X.; Gao, X.; Yi, X.; Zhang, Y.; Meng, X.; Lin, J.M. Uncovering the Metabolic Mechanism of Salidroside Alleviating Microglial Hypoxia Inflammation Based on Microfluidic Chip-Mass Spectrometry. J. Proteome Res. 2022, 21, 921–929. [Google Scholar] [CrossRef]
Panossian, A.; Seo, E.J.; Efferth, T. Novel molecular mechanisms for the adaptogenic effects of herbal extracts on isolated brain cells using systems biology. Phytomed. Int. J. Phytother. Phytopharm. 2018, 50, 257–284. [Google Scholar] [CrossRef]
Zhang, X.; Lai, W.; Ying, X.; Xu, L.; Chu, K.; Brown, J.; Chen, L.; Hong, G. Salidroside Reduces Inflammation and Brain Injury After Permanent Middle Cerebral Artery Occlusion in Rats by Regulating PI3K/PKB/Nrf2/NFκB Signaling Rather than Complement C3 Activity. Inflammation 2019, 42, 1830–1842. [Google Scholar] [CrossRef]
Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
Chen, Y.; Tang, M.; Yuan, S.; Fu, S.; Li, Y.; Li, Y.; Wang, Q.; Cao, Y.; Liu, L.; Zhang, Q. Rhodiola rosea: A Therapeutic Candidate on Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2022, 2022, 1348795. [Google Scholar] [CrossRef] [PubMed]
Zhu, L.; Wei, T.; Chang, X.; He, H.; Gao, J.; Wen, Z.; Yan, T. Effects of Salidroside on Myocardial Injury In Vivo In Vitro via Regulation of Nox/NF-κB/AP1 Pathway. Inflammation 2015, 38, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
Chen, P.; Liu, J.; Ruan, H.; Zhang, M.; Wu, P.; Yimei, D.; Han, B. Protective effects of Salidroside on cardiac function in mice with myocardial infarction. Sci. Rep. 2019, 9, 18127. [Google Scholar] [CrossRef] [Green Version]
Wei, G.; Xu, X.; Tong, H.; Wang, X.; Chen, Y.; Ding, Y.; Zhang, S.; Ju, W.; Fu, C.; Li, Z.; et al. Salidroside inhibits platelet function and thrombus formation through AKT/GSK3β signaling pathway. Aging 2020, 12, 8151–8166. [Google Scholar] [CrossRef]
Li, X.; Chen, S.; Shao, W.; Wang, S.; Yao, L. Investigating the Effects and Mechanism of Rhodiola Rosea Injection on Cardiac Function in Rats with Chronic Heart Failure. Comb. Chem. High Throughput Screen 2023, 26, 2238–2246. [Google Scholar] [CrossRef]
Li, L.; Yang, Y.; Zhang, H.; Du, Y.; Jiao, X.; Yu, H.; Wang, Y.; Lv, Q.; Li, F.; Sun, Q.; et al. Salidroside Ameliorated Intermittent Hypoxia-Aggravated Endothelial Barrier Disruption and Atherosclerosis via the cAMP/PKA/RhoA Signaling Pathway. Front. Pharm. 2021, 12, 723922. [Google Scholar] [CrossRef]
Tao, L.; Liang, Z.F.; Miao, L.; Guo, Y.J.; Li, Y.; Liu, Y.L.; Fang, D.M.; Yang, Z.J. Mechanism of salidroside against coronary artery disease by network pharmacology analysis. BMC Complement. Med. Ther. 2023, 23, 194. [Google Scholar] [CrossRef]
Zheng, T.; Bian, F.; Chen, L.; Wang, Q.; Jin, S. Beneficial Effects of Rhodiola and Salidroside in Diabetes: Potential Role of AMP-Activated Protein Kinase. Mol. Diagn. Ther. 2019, 23, 489–498. [Google Scholar] [CrossRef]
Jafari, M.; Juanson Arabit, J.G.; Courville, R.; Kiani, D.; Chaston, J.M.; Nguyen, C.D.; Jena, N.; Liu, Z.Y.; Tata, P.; Van Etten, R.A. The impact of Rhodiola rosea on biomarkers of diabetes, inflammation, and microbiota in a leptin receptor-knockout mouse model. Sci. Rep. 2022, 12, 10581. [Google Scholar] [CrossRef]
Niu, C.S.; Chen, L.J.; Niu, H.S. Antihyperglycemic action of rhodiola-aqeous extract in type1-like diabetic rats. BMC Complement. Altern. Med. 2014, 14, 20. [Google Scholar] [CrossRef] [Green Version]
Wang, M.; Luo, L.; Yao, L.; Wang, C.; Jiang, K.; Liu, X.; Xu, M.; Shen, N.; Guo, S.; Sun, C.; et al. Salidroside improves glucose homeostasis in obese mice by repressing inflammation in white adipose tissues and improving leptin sensitivity in hypothalamus. Sci. Rep. 2016, 6, 25399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Ma, Y.G.; Wang, J.W.; Zhang, Y.B.; Wang, B.F.; Dai, Z.J.; Xie, M.J.; Kang, H.F. Salidroside improved cerebrovascular vasodilation in streptozotocin-induced diabetic rats through restoring the function of BK(Ca) channel in smooth muscle cells. Cell Tissue Res. 2017, 370, 365–377. [Google Scholar] [CrossRef] [PubMed]
Shi, J.; Zhao, Q.; Hao, D.D.; Miao, H.X.; Wan, S.; Zhou, C.H.; Wang, S.Y.; Chen, S.Y.; Shang, J.; Feng, T.H. Gut microbiota profiling revealed the regulating effects of salidroside on iron metabolism in diabetic mice. Front. Endocrinol. 2022, 13, 1014577. [Google Scholar] [CrossRef] [PubMed]
Ju, L.; Wen, X.; Wang, C.; Wei, Y.; Peng, Y.; Ding, Y.; Feng, L.; Shu, L. Salidroside, A Natural Antioxidant, Improves β-Cell Survival and Function via Activating AMPK Pathway. Front. Pharm. 2017, 8, 749. [Google Scholar] [CrossRef] [Green Version]
Zhao, D.; Sun, X.; Lv, S.; Sun, M.; Guo, H.; Zhai, Y.; Wang, Z.; Dai, P.; Zheng, L.; Ye, M.; et al. Salidroside attenuates oxidized low-density lipoprotein-induced endothelial cell injury via promotion of the AMPK/SIRT1 pathway. Int. J. Mol. Med. 2019, 43, 2279–2290. [Google Scholar] [CrossRef] [Green Version]
Zhou, Q.; Han, X.; Li, R.; Zhao, W.; Bai, B.; Yan, C.; Dong, X. Anti-atherosclerosis of oligomeric proanthocyanidins from Rhodiola rosea on rat model via hypolipemic, antioxidant, anti-inflammatory activities together with regulation of endothelial function. Phytomed. Int. J. Phytother. Phytopharm. 2018, 51, 171–180. [Google Scholar] [CrossRef]
Li, F.; Tang, H.; Xiao, F.; Gong, J.; Peng, Y.; Meng, X. Protective effect of salidroside from Rhodiolae Radix on diabetes-induced oxidative stress in mice. Molecules 2011, 16, 9912. [Google Scholar] [CrossRef] [Green Version]
Wang, Z.S.; Gao, F.; Lu, F.E. Effect of ethanol extract of Rhodiola rosea on the early nephropathy in type 2 diabetic rats. J. Huazhong Univ. Sci. Technol. Med. Sci. 2013, 33, 375–378. [Google Scholar] [CrossRef]
Neagu, M.; Constantin, C.; Popescu, I.D.; Zipeto, D.; Tzanakakis, G.; Nikitovic, D.; Fenga, C.; Stratakis, C.A.; Spandidos, D.A.; Tsatsakis, A.M. Inflammation and Metabolism in Cancer Cell-Mitochondria Key Player. Front. Oncol. 2019, 9, 348. [Google Scholar] [CrossRef] [Green Version]
Kroemer, G.; Pouyssegur, J. Tumor cell metabolism: Cancer’s Achilles’ heel. Cancer Cell 2008, 13, 472–482. [Google Scholar] [CrossRef] [PubMed]
Blackadar, C.B. Historical review of the causes of cancer. World J. Clin. Oncol. 2016, 7, 54–86. [Google Scholar] [CrossRef] [PubMed]
Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2019, 21, 85–100. [Google Scholar] [CrossRef] [PubMed]
Lebelo, M.T.; Joubert, A.M.; Visagie, M.H. Warburg effect and its role in tumourigenesis. Arch. Pharm. Res. 2019, 42, 833–847. [Google Scholar] [CrossRef]
Weinberg, F.; Ramnath, N.; Nagrath, D. Reactive Oxygen Species in the Tumor Microenvironment: An Overview. Cancers 2019, 11, 1191. [Google Scholar] [CrossRef] [Green Version]
Chiche, J.; Brahimi-Horn, M.C.; Pouyssegur, J. Tumour hypoxia induces a metabolic shift causing acidosis: A common feature in cancer. J. Cell. Mol. Med. 2010, 14, 771–794. [Google Scholar] [CrossRef] [Green Version]
Zaidi, N.; Lupien, L.; Kuemmerle, N.B.; Kinlaw, W.B.; Swinnen, J.V.; Smans, K. Lipogenesis and lipolysis: The pathways exploited by the cancer cells to acquire fatty acids. Prog. Lipid Res. 2013, 52, 585–589. [Google Scholar] [CrossRef] [Green Version]
Lu, S.; Wang, Y. Nonmetabolic functions of metabolic enzymes in cancer development. Cancer Commun. 2018, 38, 63. [Google Scholar] [CrossRef] [Green Version]
Lee, N.; Kim, D. Cancer Metabolism: Fueling More than Just Growth. Mol. Cells 2016, 39, 847–854. [Google Scholar] [CrossRef] [Green Version]
Vegliante, R.; Di Leo, L.; Ciccarone, F.; Ciriolo, M.R. Hints on ATGL implications in cancer: Beyond bioenergetic clues. Cell Death Dis. 2018, 9, 316. [Google Scholar] [CrossRef] [Green Version]
Seyfried, T.N.; Flores, R.E.; Poff, A.M.; D’Agostino, D.P. Cancer as a metabolic disease: Implications for novel therapeutics. Carcinogenesis 2014, 35, 515–527. [Google Scholar] [CrossRef] [PubMed]
Zhong, H.; Xiao, M.; Zarkovic, K.; Zhu, M.; Sa, R.; Lu, J.; Tao, Y.; Chen, Q.; Xia, L.; Cheng, S.; et al. Mitochondrial control of apoptosis through modulation of cardiolipin oxidation in hepatocellular carcinoma: A novel link between oxidative stress and cancer. Free. Radic. Biol. Med. 2017, 102, 67–76. [Google Scholar] [CrossRef] [PubMed]
Kiebish, M.A.; Han, X.; Cheng, H.; Chuang, J.H.; Seyfried, T.N. Cardiolipin and electron transport chain abnormalities in mouse brain tumor mitochondria: Lipidomic evidence supporting the Warburg theory of cancer. J. Lipid Res. 2008, 49, 2545–2556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Zhu, X.; Liu, D.; Wang, Y.; Dong, M. Salidroside suppresses nonsmall cell lung cancer cells proliferation and migration via microRNA-103-3p/Mzb1. Anti-Cancer Drugs 2020, 31, 663–671. [Google Scholar] [CrossRef] [PubMed]
Radomska-Leśniewska, D.M.; Skopiński, P.; Bałan, B.J.; Białoszewska, A.; Jóźwiak, J.; Rokicki, D.; Skopińska-Różewska, E.; Borecka, A.; Hevelke, A. Angiomodulatory properties of Rhodiola spp. and other natural antioxidants. Cent.-Eur. J. Immunol. 2015, 40, 249–262. [Google Scholar] [CrossRef] [PubMed]
Wang, J.; Li, J.Z.; Lu, A.X.; Zhang, K.F.; Li, B.J. Anticancer effect of salidroside on A549 lung cancer cells through inhibition of oxidative stress and phospho-p38 expression. Oncol. Lett. 2014, 7, 1159–1164. [Google Scholar] [CrossRef]
Song, D.; Zhao, M.; Feng, L.; Wang, P.; Li, Y.; Li, W. Salidroside attenuates acute lung injury via inhibition of inflammatory cytokine production. Biomed. Pharm. Biomed. Pharm. 2021, 142, 111949. [Google Scholar] [CrossRef]
Zhang, X.; Zhu, J.; Yan, J.; Xiao, Y.; Yang, R.; Huang, R.; Zhou, J.; Wang, Z.; Xiao, W.; Zheng, C.; et al. Systems pharmacology unravels the synergic target space and therapeutic potential of Rhodiola rosea L. for non-small cell lung cancer. Phytomed. Int. J. Phytother. Phytopharm. 2020, 79, 153326. [Google Scholar] [CrossRef]
Liu, Z.; Li, X.; Simoneau, A.R.; Jafari, M.; Zi, X. Rhodiola rosea extracts and salidroside decrease the growth of bladder cancer cell lines via inhibition of the mTOR pathway and induction of autophagy. Mol. Carcinog. 2012, 51, 257–267. [Google Scholar] [CrossRef] [Green Version]
Bocharova, O.A.; Matveev, B.P.; Baryshnikov, A.; Figurin, K.M.; Serebriakova, R.V.; Bodrova, N.B. The effect of a Rhodiola rosea extract on the incidence of recurrences of a superficial bladder cancer (experimental clinical research). Urol. Nefrol. 1995, 2, 46–47. [Google Scholar]
Schriner, S.E.; Lee, K.; Truong, S.; Salvadora, K.T.; Maler, S.; Nam, A.; Lee, T.; Jafari, M. Extension of Drosophila lifespan by Rhodiola rosea through a mechanism independent from dietary restriction. PLoS ONE 2013, 8, e63886. [Google Scholar] [CrossRef] [Green Version]
Wiegant, F.A.; Surinova, S.; Ytsma, E.; Langelaar-Makkinje, M.; Wikman, G.; Post, J.A. Plant adaptogens increase lifespan and stress resistance in C. elegans. Biogerontology 2009, 10, 27–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Yokoyama, N.N.; Denmon, A.; Uchio, E.M.; Jordan, M.; Mercola, D.; Zi, X. When Anti-Aging Studies Meet Cancer Chemoprevention: Can Anti-Aging Agent Kill Two Birds with One Blow? Curr. Pharm. Rep. 2015, 1, 420–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Kong, Y.H.; Xu, S.P. Salidroside prevents skin carcinogenesis induced by DMBA/TPA in a mouse model through suppression of inflammation and promotion of apoptosis. Oncol. Rep. 2018, 39, 2513–2526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Ma, W.; Wang, Z.; Zhao, Y.; Wang, Q.; Zhang, Y.; Lei, P.; Lu, W.; Yan, S.; Zhou, J.; Li, X.; et al. Salidroside Suppresses the Proliferation and Migration of Human Lung Cancer Cells through AMPK-Dependent NLRP3 Inflammasome Regulation. Oxid. Med. Cell. Longev. 2021, 2021, 6614574. [Google Scholar] [CrossRef] [PubMed]
El-Kott, A.F.; ElBealy, E.R.; Alshehri, A.S.; El-Kenawy, A.E.; Khalifa, H.S.; AlRamlawy, A.M. Salidroside induces cell apoptosis and inhibits the invasiveness of HT29 colorectal cells by regulating protein kinase R, NF-κB and STAT3. Cancer Biomark. Sect. Dis. Markers 2021, 31, 13–25. [Google Scholar] [CrossRef] [PubMed]
Sun, A.Q.; Ju, X.L. Inhibitory effects of salidroside on MCF-7 breast cancer cells in vivo. J. Int. Med. Res. 2020, 48, 300060520968353. [Google Scholar] [CrossRef]
Yuetong, L.; Shangzhu, L.; Qinglin, H.; Pingping, H. Salidroside inhibits proliferation, migration and invasion of human pancreatic cancer PANC1 and SW1990 cells through the AKT and ERK signaling pathway. Die Pharm. 2020, 75, 385–388. [Google Scholar] [CrossRef]
Rong, L.; Li, Z.; Leng, X.; Li, H.; Ma, Y.; Chen, Y.; Song, F. Salidroside induces apoptosis and protective autophagy in human gastric cancer AGS cells through the PI3K/Akt/mTOR pathway. Biomed. Pharmacother. Biomed. Pharmacother. 2020, 122, 109726. [Google Scholar] [CrossRef]
Huang, L.; Huang, Z.; Lin, W.; Wang, L.; Zhu, X.; Chen, X.; Yang, S.; Lv, C. Salidroside suppresses the growth and invasion of human osteosarcoma cell lines MG63 and U2OS in vitro by inhibiting the JAK2/STAT3 signaling pathway. Int. J. Oncol. 2019, 54, 1969–1980. [Google Scholar] [CrossRef]
Kim, H.; Kong, C.S.; Seo, Y. Salidroside, 8(E)-Nuezhenide, and Ligustroside from Ligustrum japonicum Fructus Inhibit Expressions of MMP-2 and -9 in HT 1080 Fibrosarcoma. Int. J. Mol. Sci. 2022, 23, 2660. [Google Scholar] [CrossRef] [PubMed]
Liu, S.; Li, Y.; Li, Z. Salidroside suppresses the activation of nasopharyngeal carcinoma cells via targeting miR-4262/GRP78 axis. Cell Cycle 2022, 21, 720–729. [Google Scholar] [CrossRef] [PubMed]
Zeng, Q.; Nie, X.; Li, L.; Liu, H.F.; Peng, Y.Y.; Zhou, W.T.; Hu, X.J.; Xu, X.Y.; Chen, X.L. Salidroside Promotes Sensitization to Doxorubicin in Human Cancer Cells by Affecting the PI3K/Akt/HIF Signal Pathway and Inhibiting the Expression of Tumor-Resistance-Related Proteins. J. Nat. Prod. 2022, 85, 196–204. [Google Scholar] [CrossRef] [PubMed]
Chen, D.; Luo, C. Salidroside inhibits chronic myeloid leukemia cell proliferation and induces apoptosis by regulating the miR-140-5p/wnt5a/β-catenin axis. Exp. Ther. Med. 2021, 22, 1249. [Google Scholar] [CrossRef] [PubMed]
Dai, Z.; Zhang, X.; Li, W.; Tang, J.; Pan, T.; Ma, C.; Guan, Q. Salidroside Induces Apoptosis in Human Gastric Cancer Cells via the Downregulation of ENO1/PKM2/GLUT1 Expression. Biol. Pharm. Bull. 2021, 44, 1724–1731. [Google Scholar] [CrossRef]
Fan, X.J.; Wang, Y.; Wang, L.; Zhu, M. Salidroside induces apoptosis and autophagy in human colorectal cancer cells through inhibition of PI3K/Akt/mTOR pathway. Oncol. Rep. 2016, 36, 3559–3567. [Google Scholar] [CrossRef] [Green Version]
Yu, G.; Li, N.; Zhao, Y.; Wang, W.; Feng, X.L. Salidroside induces apoptosis in human ovarian cancer SKOV3 and A2780 cells through the p53 signaling pathway. Oncol. Lett. 2018, 15, 6513–6518. [Google Scholar] [CrossRef] [Green Version]
Lu, L.; Liu, S.; Dong, Q.; Xin, Y. Salidroside suppresses the metastasis of hepatocellular carcinoma cells by inhibiting the activation of the Notch1 signaling pathway. Mol. Med. Rep. 2019, 19, 4964–4972. [Google Scholar] [CrossRef]
Xin, X.; Yao, D.; Zhang, K.; Han, S.; Liu, D.; Wang, H.; Liu, X.; Li, G.; Huang, J.; Wang, J. Protective effects of Rosavin on bleomycin-induced pulmonary fibrosis via suppressing fibrotic and inflammatory signaling pathways in mice. Biomed. Pharm. Biomed. Pharm. 2019, 115, 108870. [Google Scholar] [CrossRef] [PubMed]
Marchev, A.S.; Dimitrova, P.; Koycheva, I.K.; Georgiev, M.I. Altered expression of TRAIL on mouse T cells via ERK phosphorylation by Rhodiola rosea L. and its marker compounds. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 108, 419–428. [Google Scholar] [CrossRef]
Chen, Chung-Yu, Chien-Wen Hou, Jeffrey R. Bernard, Chiu-Chou Chen, Ta-Cheng Hung, Lu-Ling Cheng, Yi-Hung Liao, and Chia-Hua Kuo. “Rhodiola Crenulata- and Cordyceps Sinensis-Based Supplement Boosts Aerobic Exercise Performance after Short-Term High Altitude Training.” High Altitude Medicine & Biology 15, no. 3 (September 1, 2014): 371–79. https://doi.org/10.1089/ham.2013.1114. ↵
Parisi, A., E. Tranchita, G. Duranti, E. Ciminelli, F. Quaranta, R. Ceci, C. Cerulli, P. Borrione, and S. Sabatini. “Effects of Chronic Rhodiola Rosea Supplementation on Sport Performance and Antioxidant Capacity in Trained Male: Preliminary Results.” The Journal of Sports Medicine and Physical Fitness 50, no. 1 (March 2010): 57–63. ↵
Shanely, R. Andrew, David C. Nieman, Kevin A. Zwetsloot, Amy M. Knab, Hidetaka Imagita, Beibei Luo, Barbara Davis, and José M. Zubeldia. “Evaluation of Rhodiola Rosea Supplementation on Skeletal Muscle Damage and Inflammation in Runners Following a Competitive Marathon.” Brain, Behavior, and Immunity 39 (July 2014): 204–10. https://doi.org/10.1016/j.bbi.2013.09.005. ↵
Gray, Beverley. The Boreal Herbal: Wild Food and Medicine Plants of the North. Whitehorse, Yukon: Aroma Borealis Press, 2011. ↵
Winston, David, and Steven Maimes. Adaptogens: Herbs for Strength, Stamina, and Stress Relief. Rochester, VT: Healing Arts Press, 2007. ↵
Keller, Amy C. “RE: Review of Rhodiola in the Treatment of Depression.” American Botanical Council. December 15, 2016. Accessed January 21, 2019. ↵
Mao, Jun J., Sharon X. Xie, Jarcy Zee, Irene Soeller, Qing S. Li, Kenneth Rockwell, and Jay D. Amsterdam. “Rhodiola Rosea versus Sertraline for Major Depressive Disorder: A Randomized Placebo-Controlled Trial.” Phytomedicine: International Journal of Phytotherapy and Phytopharmacology 22, no. 3 (March 15, 2015): 394–99. https://doi.org/10.1016/j.phymed.2015.01.010. ↵
Huang, Li-Yen, I.-Chuan Yen, Wei-Cheng Tsai, Blerina Ahmetaj-Shala, Tsu-Chung Chang, Chien-Sung Tsai, and Shih-Yu Lee. “Rhodiola Crenulata Attenuates High Glucose Induced Endothelial Dysfunction in Human Umbilical Vein Endothelial Cells.” The American Journal of Chinese Medicine 45, no. 6 (2017): 1201–16. https://doi.org/10.1142/S0192415X17500665. ↵
Chuang, Ming-Lung, Tzu-Chin Wu, Yau-Tung Wang, Yau-Chen Wang, Thomas C.-Y. Tsao, James Cheng-Chung Wei, Chia-Yin Chen, and I-Feng Lin. “Adjunctive Treatment with Rhodiola Crenulata in Patients with Chronic Obstructive Pulmonary Disease – A Randomized Placebo Controlled Double Blind Clinical Trial.” PLoS ONE10, no. 6 (June 22, 2015). https://doi.org/10.1371/journal.pone.0128142. ↵
Senthilkumar, Ravichandran, Thangaraj Parimelazhagan, Om Prakash Chaurasia, and R. B. Srivastava. “Free Radical Scavenging Property and Antiproliferative Activity of Rhodiola Imbricata Edgew Extracts in HT-29 Human Colon Cancer Cells.” Asian Pacific Journal of Tropical Medicine 6, no. 1 (January 2013): 11–19. https://doi.org/10.1016/S1995-7645(12)60194-1. ↵
Li, Hai, and Chen Chen. “Inhibition of Autophagy Enhances Synergistic Effects of Salidroside and Anti-Tumor Agents against Colorectal Cancer.” BMC Complementary and Alternative Medicine 17 (December 16, 2017). https://doi.org/10.1186/s12906-017-2046-z. ↵
Zeng, Wei, Tao Xiao, Anlie Cai, Weiliang Cai, Huanhuan Liu, Jingling Liu, Jie Li, et al. “Inhibiting ROS-TFEB-Dependent Autophagy Enhances Salidroside-Induced Apoptosis in Human Chondrosarcoma Cells.” Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology 43, no. 4 (2017): 1487–1502. https://doi.org/10.1159/000481971. ↵
MÃjÃtez, Salvador, Rosa-MarÃna Giner, and Mari-Carmen Recio. “Immunmodulatory and Antiproliferative Properties of Rhodiola Species.” Planta Medica 82, no. 11/12 (July 2016): 952–60. https://doi.org/10.1055/s-0042-107254. ↵
Loo, Wings T. Y., L. J. Jin, Louis W. C. Chow, Mary N. B. Cheung, and Min Wang. “Rhodiola Algida Improves Chemotherapy-Induced Oral Mucositis in Breast Cancer Patients.” Expert Opinion on Investigational Drugs 19 Suppl 1 (April 2010): S91-100. https://doi.org/10.1517/13543781003727057. ↵
Booker, Anthony, Banaz Jalil, Debora Frommenwiler, Eike Reich, Lixiang Zhai, Zarko Kulic, and Michael Heinrich. “The Authenticity and Quality of Rhodiola Rosea Products.” Phytomedicine: International Journal of Phytotherapy and Phytopharmacology 23, no. 7 (June 15, 2016): 754–62. https://doi.org/10.1016/j.phymed.2015.10.006. ↵
Winston, David, and Steven Maimes. Adaptogens: Herbs for Strength, Stamina, and Stress Relief. Rochester, VT: Healing Arts Press, 2007. ↵
The above post was the 25th installment of a series titled Stacking Functions in the Garden, Food Forest and Medicine Cabinet : The Regenerative Way From Seed To Apothecary.








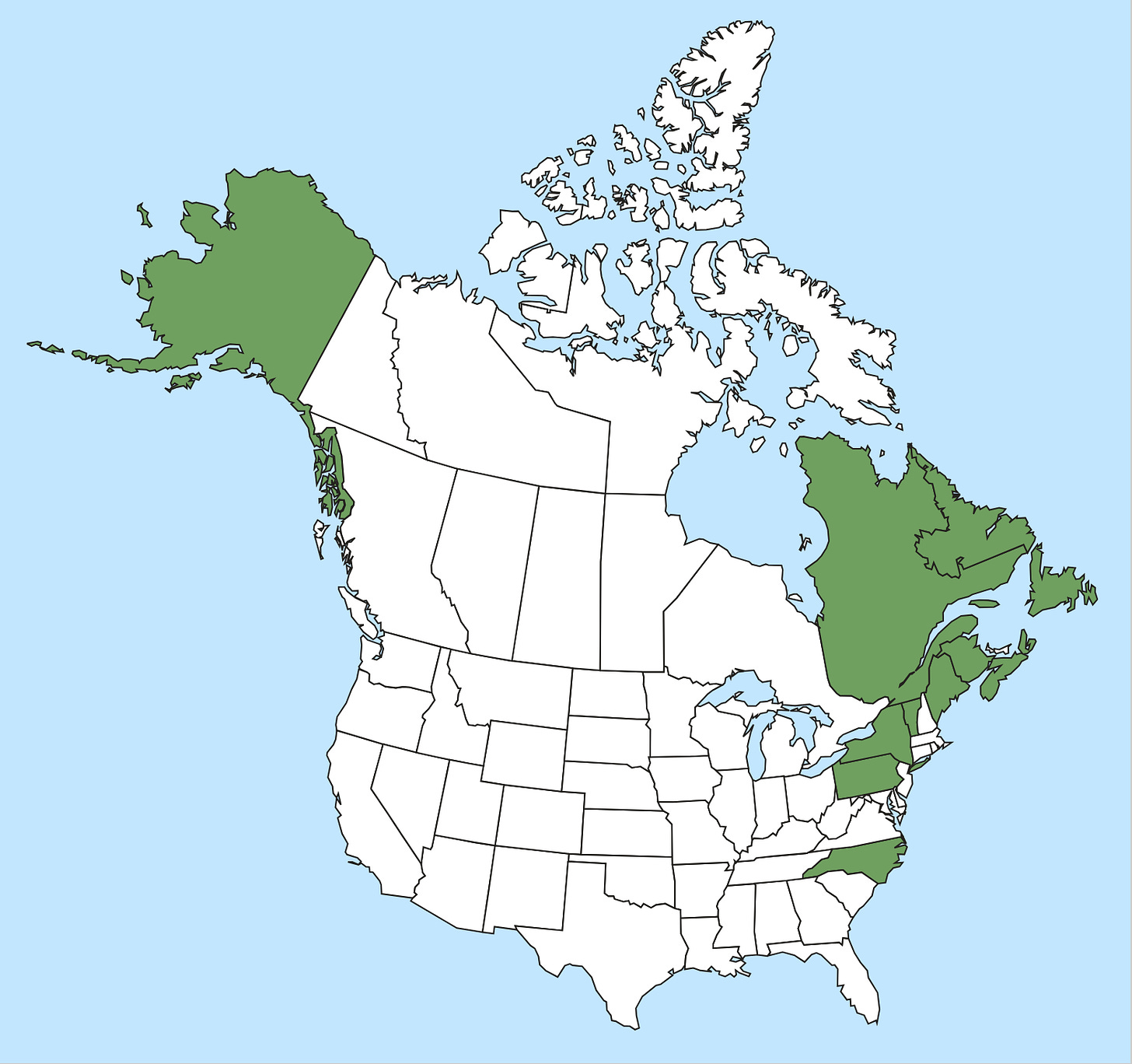

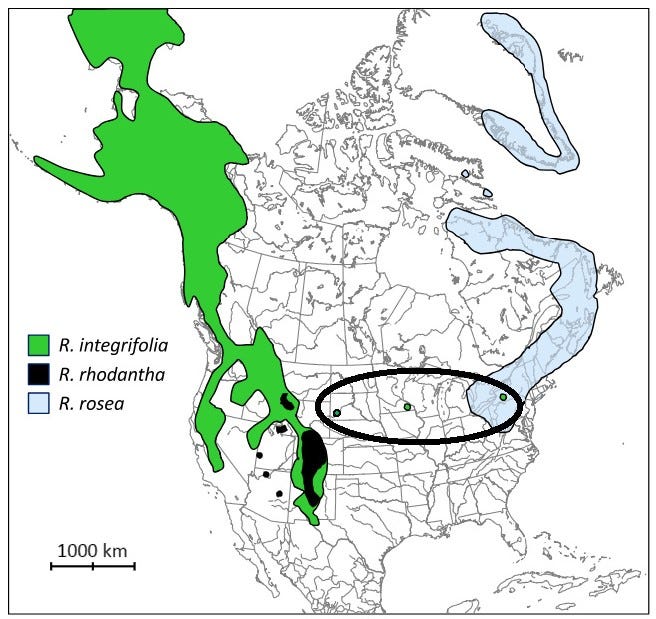


































The earth doesn't need AI when wonderful minds and abilities like yours exist!
We haven't even begun to tap our own intelligence and abilities, yet there are those who think a technological 'mind' is needed because ours aren't good enough.
Very comprehensive. i have taken some of these before. i like them better that maca, for energy. I had no idea they were so pretty, and now i am inspired to grow them.